From Wikipedia, the free encyclopedia
Polyphenol compound
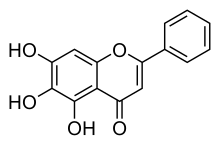
Baicalein (5,6,7-trihydroxyflavone) is a
flavone , a type of
flavonoid ,
[1] originally isolated from the roots of
Scutellaria baicalensis and
Scutellaria lateriflora . It is also a constituent of
Oroxylum indicum (Indian trumpetflower) and
thyme .
[2] It is the
aglycone of
baicalin .
Pharmacology Baicalein, along with its
glucuronide
baicalin , is a
positive allosteric modulator of the
benzodiazepine site and a non-benzodiazepine site of the
GABAA receptor , but with an affinity over 250× lower than
diazepam .
[3]
[4]
[5] It displays subtype selectivity for
α2 and
α3 subunit -containing GABAA receptors.
[6]
The flavonoid has been shown to inhibit certain types of
lipoxygenases .
[7]
Baicalein is an
inhibitor of
CYP2C9 ,
[8] an
enzyme of the
cytochrome P450 system that
metabolizes
drugs in the body.
A derivative of baicalin is a known
prolyl endopeptidase inhibitor.
[9]
See also References
^
"Flavonoids" . Micronutrient Information Center, Linus Pauling Institute, Oregon State University. 2024. Retrieved 9 May 2024 .
^ Matsumoto T (2008).
Phytochemistry Research Progress ISBN
9781604562323
^ Zhang SQ, Obregon D, Ehrhart J, Deng J, Tian J, Hou H, et al. (September 2013).
"Baicalein reduces β-amyloid and promotes nonamyloidogenic amyloid precursor protein processing in an Alzheimer's disease transgenic mouse model" . Journal of Neuroscience Research . 91 (9): 1239–1246.
doi :
10.1002/jnr.23244 .
PMC
3810722 .
PMID
23686791 .
^ Liao JF, Wang HH, Chen MC, Chen CC, Chen CF (August 1998). "Benzodiazepine binding site-interactive flavones from Scutellaria baicalensis root". Planta Medica . 64 (6): 571–572.
doi :
10.1055/s-2006-957517 .
PMID
9776664 .
S2CID
260251315 .
^ Roberts AA (2004). "Testing efficacy of natural anxiolytic compounds". In Cooper EL, Yamaguchi N (eds.). Complementary and Alternative Approaches to Biomedicine . Advances in Experimental Medicine and Biology. Vol. 546. pp. 181–191.
doi :
10.1007/978-1-4757-4820-8_13 .
ISBN
978-1-4419-3441-3 PMID
15584374 .
^ Wang F, Xu Z, Ren L, Tsang SY, Xue H (December 2008). "GABA A receptor subtype selectivity underlying selective anxiolytic effect of baicalin". Neuropharmacology . 55 (7): 1231–1237.
doi :
10.1016/j.neuropharm.2008.07.040 .
PMID
18723037 .
S2CID
20133964 .
^ Deschamps JD, Kenyon VA, Holman TR (June 2006).
"Baicalein is a potent in vitro inhibitor against both reticulocyte 15-human and platelet 12-human lipoxygenases" . Bioorganic & Medicinal Chemistry . 14 (12): 4295–4301.
doi :
10.1016/j.bmc.2006.01.057 .
PMID
16500106 .
S2CID
645610 .
^ Si D, Wang Y, Zhou YH, Guo Y, Wang J, Zhou H, et al. (March 2009).
"Mechanism of CYP2C9 inhibition by flavones and flavonols" (PDF) . Drug Metabolism and Disposition . 37 (3): 629–634.
doi :
10.1124/dmd.108.023416 .
PMID
19074529 .
S2CID
285706 . Archived from
the original (PDF) on 2008-12-17. Retrieved 2009-02-19 .
^ Tarragó T, Kichik N, Claasen B, Prades R, Teixidó M, Giralt E (August 2008). "Baicalin, a prodrug able to reach the CNS, is a prolyl oligopeptidase inhibitor". Bioorganic & Medicinal Chemistry . 16 (15): 7516–7524.
doi :
10.1016/j.bmc.2008.04.067 .
PMID
18650094 .
Aglycones
Monohydroxyflavone Dihydroxyflavones Trihydroxyflavones Tetrahydroxyflavones Pentahydroxyflavones O-methylated flavones
Glycosides
of apigenin of baicalein of hypolaetin of luteolin
Acetylated Sulfated glycosides Polymers Drugs
Alcohols
Barbiturates
Benzodiazepines
Carbamates
Flavonoids
Imidazoles
Kava constituents
Monoureides
Neuroactive steroids
Nonbenzodiazepines
Phenols
Piperidinediones
Pyrazolopyridines
Quinazolinones
Volatiles /
gases Others/unsorted
3-Hydroxybutanal
α-EMTBL
AA-29504
Alogabat
Avermectins (e.g.,
ivermectin )
Bromide compounds (e.g.,
lithium bromide ,
potassium bromide ,
sodium bromide )
Carbamazepine
Chloralose
Chlormezanone
Clomethiazole
Darigabat
DEABL
Deuterated etifoxine
Dihydroergolines (e.g.,
dihydroergocryptine ,
dihydroergosine ,
dihydroergotamine ,
ergoloid (dihydroergotoxine) )
DS2
Efavirenz
Etazepine
Etifoxine
Fenamates (e.g.,
flufenamic acid ,
mefenamic acid ,
niflumic acid ,
tolfenamic acid )
Fluoxetine
Flupirtine
Hopantenic acid
KRM-II-81
Lanthanum
Lavender oil
Lignans (e.g.,
4-O-methylhonokiol ,
honokiol ,
magnolol ,
obovatol )
Loreclezole
Menthyl isovalerate (validolum)
Monastrol
Niacin
Niacinamide
Org 25,435
Phenytoin
Propanidid
Retigabine (ezogabine)
Safranal
Seproxetine
Stiripentol
Sulfonylalkanes (e.g.,
sulfonmethane (sulfonal) ,
tetronal ,
trional )
Terpenoids (e.g.,
borneol )
Topiramate
Valerian constituents (e.g.,
isovaleric acid ,
isovaleramide ,
valerenic acid ,
valerenol )
ER Tooltip Estrogen receptor
Agonists
Steroidal:
2-Hydroxyestradiol
2-Hydroxyestrone
3-Methyl-19-methyleneandrosta-3,5-dien-17β-ol
3α-Androstanediol
3α,5α-Dihydrolevonorgestrel
3β,5α-Dihydrolevonorgestrel
3α-Hydroxytibolone
3β-Hydroxytibolone
3β-Androstanediol
4-Androstenediol
4-Androstenedione
4-Fluoroestradiol
4-Hydroxyestradiol
4-Hydroxyestrone
4-Methoxyestradiol
4-Methoxyestrone
5-Androstenediol
7-Oxo-DHEA
7α-Hydroxy-DHEA
7α-Methylestradiol
7β-Hydroxyepiandrosterone
8,9-Dehydroestradiol
8,9-Dehydroestrone
8β-VE2
10β,17β-Dihydroxyestra-1,4-dien-3-one (DHED)
11β-Chloromethylestradiol
11β-Methoxyestradiol
15α-Hydroxyestradiol
16-Ketoestradiol
16-Ketoestrone
16α-Fluoroestradiol
16α-Hydroxy-DHEA
16α-Hydroxyestrone
16α-Iodoestradiol
16α-LE2
16β-Hydroxyestrone
16β,17α-Epiestriol (16β-hydroxy-17α-estradiol)
17α-Estradiol (
alfatradiol )
17α-Dihydroequilenin
17α-Dihydroequilin
17α-Epiestriol (16α-hydroxy-17α-estradiol)
17α-Ethynyl-3α-androstanediol
17α-Ethynyl-3β-androstanediol
17β-Dihydroequilenin
17β-Dihydroequilin
17β-Methyl-17α-dihydroequilenin
Abiraterone
Abiraterone acetate
Alestramustine
Almestrone
Anabolic steroids (e.g.,
testosterone and
esters ,
methyltestosterone ,
metandienone (methandrostenolone) ,
nandrolone and
esters , many others; via estrogenic metabolites)
Atrimustine
Bolandiol
Bolandiol dipropionate
Butolame
Clomestrone
Cloxestradiol
Conjugated estriol
Conjugated estrogens
Cyclodiol
Cyclotriol
DHEA
DHEA-S
ent -Estradiol
Epiestriol (16β-epiestriol, 16β-hydroxy-17β-estradiol)
Epimestrol
Equilenin
Equilin
ERA-63 (ORG-37663)
Esterified estrogens
Estetrol
Estradiol
Estramustine
Estramustine phosphate
Estrapronicate
Estrazinol
Estriol
Estrofurate
Estrogenic substances
Estromustine
Estrone
Etamestrol (eptamestrol)
Ethinylandrostenediol
Ethinylestradiol
Ethinylestriol
Ethylestradiol
Etynodiol
Etynodiol diacetate
Hexolame
Hippulin
Hydroxyestrone diacetate
Lynestrenol
Lynestrenol phenylpropionate
Mestranol
Methylestradiol
Moxestrol
Mytatrienediol
Nilestriol
Norethisterone
Noretynodrel
Orestrate
Pentolame
Prodiame
Prolame
Promestriene
RU-16117
Quinestradol
Quinestrol
Tibolone Xenoestrogens:
Anise -related (e.g.,
anethole ,
anol ,
dianethole ,
dianol ,
photoanethole )
Chalconoids (e.g.,
isoliquiritigenin ,
phloretin ,
phlorizin (phloridzin) ,
wedelolactone )
Coumestans (e.g.,
coumestrol ,
psoralidin )
Flavonoids (incl.
7,8-DHF ,
8-prenylnaringenin ,
apigenin ,
baicalein ,
baicalin ,
biochanin A ,
calycosin ,
catechin ,
daidzein ,
daidzin ,
ECG ,
EGCG ,
epicatechin ,
equol ,
formononetin ,
glabrene ,
glabridin ,
genistein ,
genistin ,
glycitein ,
kaempferol ,
liquiritigenin ,
mirificin ,
myricetin ,
naringenin ,
penduletin ,
pinocembrin ,
prunetin ,
puerarin ,
quercetin ,
tectoridin ,
tectorigenin )
Lavender oil
Lignans (e.g.,
enterodiol ,
enterolactone ,
nyasol (cis -hinokiresinol) )
Metalloestrogens (e.g.,
cadmium )
Pesticides (e.g.,
alternariol ,
dieldrin ,
endosulfan ,
fenarimol ,
HPTE ,
methiocarb ,
methoxychlor ,
triclocarban ,
triclosan )
Phytosteroids (e.g.,
digitoxin (
digitalis ),
diosgenin ,
guggulsterone )
Phytosterols (e.g.,
β-sitosterol ,
campesterol ,
stigmasterol )
Resorcylic acid lactones (e.g.,
zearalanone ,
α-zearalenol ,
β-zearalenol ,
zearalenone ,
zeranol (α-zearalanol) ,
taleranol (teranol, β-zearalanol) )
Steroid -like (e.g.,
deoxymiroestrol ,
miroestrol )
Stilbenoids (e.g.,
resveratrol ,
rhaponticin )
Synthetic xenoestrogens (e.g.,
alkylphenols ,
bisphenols (e.g.,
BPA ,
BPF ,
BPS ),
DDT ,
parabens ,
PBBs ,
PHBA ,
phthalates ,
PCBs )
Others (e.g.,
agnuside ,
rotundifuran ) Mixed
SERMs Tooltip Selective estrogen receptor modulators ) Antagonists
Coregulator-binding modulators:
ERX-11
GPER Tooltip G protein-coupled estrogen receptor
Agonists Antagonists Unknown
Receptor (
ligands )
BLT Tooltip Leukotriene B4 receptor
BLT1 Tooltip Leukotriene B4 receptor 1
BLT2 Tooltip Leukotriene B4 receptor 2
CysLT Tooltip Cysteinyl leukotriene receptor
CysLT1 Tooltip Cysteinyl leukotriene receptor 1
CysLT2 Tooltip Cysteinyl leukotriene receptor 2
CysLTE Tooltip Cysteinyl leukotriene receptor E
Enzyme (
inhibitors )
5-LOX Tooltip Arachidonate 5-lipoxygenase
12-LOX Tooltip Arachidonate 12-lipoxygenase
15-LOX Tooltip Arachidonate 15-lipoxygenase
LTA4 H Tooltip Leukotriene A4 hydrolase
LTB4 H Tooltip Leukotriene B4 ω-hydroxylase
LTC4 S Tooltip Leukotriene C4 synthase
LTC4 H Tooltip Leukotriene C4 hydrolase
LTD4 Tooltip Leukotriene D4 hydrolase
Others