From Wikipedia, the free encyclopedia
Chemical compound
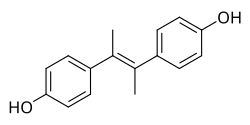
Dimethylstilbestrol (DMS ) is a
nonsteroidal estrogen of the
stilbestrol group related to
diethylstilbestrol which was never marketed.
[1]
[2]
[3]
[4]
[5] : 213 estriol and
meso -butoestrol
[6]
[7]
[8]
[9] The
affinity of DMS for the ER was reported as about 10% of that of
estradiol .
[10] For comparison,
diethylstilbestrol had 140% of the affinity of estradiol for the ER.
[10]
The
endometrial proliferation dose of DMS in women is 20 mg.
[5] : 212–213 intramuscular injection of DMS has a
duration of approximately 12 days in humans.
[5]
References
^ Pincus G (3 September 2013).
"Blastocyst Development and Implantation" . The Control of Fertility . Elsevier. pp. 126–.
ISBN
978-1-4832-7088-3
^ Emmens CW, Martin L (5 December 2016).
"Anti-Estrogens" . In Dorfman RI (ed.). Steroidal Activity in Experimental Animals and Man . Elsevier Science. pp. 83–.
ISBN
978-1-4832-7299-3
^ Hilf R, Wittliff JL (27 November 2013).
"Mechanisms of Action of Estrogens" . In Sartorelli AC, Johns DG (eds.). Antineoplastic and Immunosuppressive Agents . Springer Science & Business Media. pp. 110–.
ISBN
978-3-642-65806-8
^ Horwitz KB, McGuire WL (14 December 2013).
"Antiestrogens: Mechanism of Action and Effects in Breast Cancer" . In McGuire W (ed.). Experimental Biology . Springer Science & Business Media. pp. 169–.
ISBN
978-1-4757-4673-0 ^
a b c Knörr K, Beller FK, Lauritzen C (17 April 2013).
"Prinzipien der Hormonbehandlung: Die wichtigsten hormonalen Behandlungsmethoden" . Lehrbuch der Gynäkologie . Springer-Verlag.
ISBN
978-3-662-00942-0
^ Katzenellenbogen BS, Iwamoto HS, Heiman DF, Lan NC, Katzenellenbogen JA (1978). "Stilbestrols and stilbestrol derivatives: estrogenic potency and temporal relationships between estrogen receptor binding and uterine growth". Molecular and Cellular Endocrinology . 10 (1): 103–113.
doi :
10.1016/0303-7207(78)90063-1 .
PMID
564791 .
S2CID
45882988 .
^ Martin L (January 1969). "Dimethylstilbestrol and 16-oxo-estradiol: anti-estrogens or estrogens?". Steroids . 13 (1): 1–10.
doi :
10.1016/s0039-128x(69)80055-3 .
PMID
5764482 .
^ Emmens CW, Cox RI, Martin L (July 1959). "Oestrogen inhibitors of the stilboestrol series". The Journal of Endocrinology . 18 (4): 372–380.
doi :
10.1677/joe.0.0180372 .
PMID
13820198 .
^ Emmens CW, Cox RI (September 1958). "Dimethylstilboestrol as an oestrogen inhibitor". The Journal of Endocrinology . 17 (3): 265–271.
doi :
10.1677/joe.0.0170265 .
PMID
13587831 . ^
a b Jordan VC, Lieberman ME (September 1984). "Estrogen-stimulated prolactin synthesis in vitro. Classification of agonist, partial agonist, and antagonist actions based on structure". Molecular Pharmacology . 26 (2): 279–285.
CiteSeerX
10.1.1.1064.9508 .
PMID
6541293 .
ER Tooltip Estrogen receptor
Agonists
Steroidal:
2-Hydroxyestradiol
2-Hydroxyestrone
3-Methyl-19-methyleneandrosta-3,5-dien-17β-ol
3α-Androstanediol
3α,5α-Dihydrolevonorgestrel
3β,5α-Dihydrolevonorgestrel
3α-Hydroxytibolone
3β-Hydroxytibolone
3β-Androstanediol
4-Androstenediol
4-Androstenedione
4-Fluoroestradiol
4-Hydroxyestradiol
4-Hydroxyestrone
4-Methoxyestradiol
4-Methoxyestrone
5-Androstenediol
7-Oxo-DHEA
7α-Hydroxy-DHEA
7α-Methylestradiol
7β-Hydroxyepiandrosterone
8,9-Dehydroestradiol
8,9-Dehydroestrone
8β-VE2
10β,17β-Dihydroxyestra-1,4-dien-3-one (DHED)
11β-Chloromethylestradiol
11β-Methoxyestradiol
15α-Hydroxyestradiol
16-Ketoestradiol
16-Ketoestrone
16α-Fluoroestradiol
16α-Hydroxy-DHEA
16α-Hydroxyestrone
16α-Iodoestradiol
16α-LE2
16β-Hydroxyestrone
16β,17α-Epiestriol (16β-hydroxy-17α-estradiol)
17α-Estradiol (
alfatradiol )
17α-Dihydroequilenin
17α-Dihydroequilin
17α-Epiestriol (16α-hydroxy-17α-estradiol)
17α-Ethynyl-3α-androstanediol
17α-Ethynyl-3β-androstanediol
17β-Dihydroequilenin
17β-Dihydroequilin
17β-Methyl-17α-dihydroequilenin
Abiraterone
Abiraterone acetate
Alestramustine
Almestrone
Anabolic steroids (e.g.,
testosterone and
esters ,
methyltestosterone ,
metandienone (methandrostenolone) ,
nandrolone and
esters , many others; via estrogenic metabolites)
Atrimustine
Bolandiol
Bolandiol dipropionate
Butolame
Clomestrone
Cloxestradiol
Conjugated estriol
Conjugated estrogens
Cyclodiol
Cyclotriol
DHEA
DHEA-S
ent -Estradiol
Epiestriol (16β-epiestriol, 16β-hydroxy-17β-estradiol)
Epimestrol
Equilenin
Equilin
ERA-63 (ORG-37663)
Esterified estrogens
Estetrol
Estradiol
Estramustine
Estramustine phosphate
Estrapronicate
Estrazinol
Estriol
Estrofurate
Estrogenic substances
Estromustine
Estrone
Etamestrol (eptamestrol)
Ethinylandrostenediol
Ethinylestradiol
Ethinylestriol
Ethylestradiol
Etynodiol
Etynodiol diacetate
Hexolame
Hippulin
Hydroxyestrone diacetate
Lynestrenol
Lynestrenol phenylpropionate
Mestranol
Methylestradiol
Moxestrol
Mytatrienediol
Nilestriol
Norethisterone
Noretynodrel
Orestrate
Pentolame
Prodiame
Prolame
Promestriene
RU-16117
Quinestradol
Quinestrol
Tibolone Xenoestrogens:
Anise -related (e.g.,
anethole ,
anol ,
dianethole ,
dianol ,
photoanethole )
Chalconoids (e.g.,
isoliquiritigenin ,
phloretin ,
phlorizin (phloridzin) ,
wedelolactone )
Coumestans (e.g.,
coumestrol ,
psoralidin )
Flavonoids (incl.
7,8-DHF ,
8-prenylnaringenin ,
apigenin ,
baicalein ,
baicalin ,
biochanin A ,
calycosin ,
catechin ,
daidzein ,
daidzin ,
ECG ,
EGCG ,
epicatechin ,
equol ,
formononetin ,
glabrene ,
glabridin ,
genistein ,
genistin ,
glycitein ,
kaempferol ,
liquiritigenin ,
mirificin ,
myricetin ,
naringenin ,
penduletin ,
pinocembrin ,
prunetin ,
puerarin ,
quercetin ,
tectoridin ,
tectorigenin )
Lavender oil
Lignans (e.g.,
enterodiol ,
enterolactone ,
nyasol (cis -hinokiresinol) )
Metalloestrogens (e.g.,
cadmium )
Pesticides (e.g.,
alternariol ,
dieldrin ,
endosulfan ,
fenarimol ,
HPTE ,
methiocarb ,
methoxychlor ,
triclocarban ,
triclosan )
Phytosteroids (e.g.,
digitoxin (
digitalis ),
diosgenin ,
guggulsterone )
Phytosterols (e.g.,
β-sitosterol ,
campesterol ,
stigmasterol )
Resorcylic acid lactones (e.g.,
zearalanone ,
α-zearalenol ,
β-zearalenol ,
zearalenone ,
zeranol (α-zearalanol) ,
taleranol (teranol, β-zearalanol) )
Steroid -like (e.g.,
deoxymiroestrol ,
miroestrol )
Stilbenoids (e.g.,
resveratrol ,
rhaponticin )
Synthetic xenoestrogens (e.g.,
alkylphenols ,
bisphenols (e.g.,
BPA ,
BPF ,
BPS ),
DDT ,
parabens ,
PBBs ,
PHBA ,
phthalates ,
PCBs )
Others (e.g.,
agnuside ,
rotundifuran ) Mixed
SERMs Tooltip Selective estrogen receptor modulators ) Antagonists
Coregulator-binding modulators:
ERX-11
GPER Tooltip G protein-coupled estrogen receptor
Agonists Antagonists Unknown