| |
| Names | |
|---|---|
|
Preferred IUPAC name
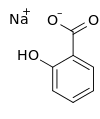
Sodium 2-hydroxybenzoate | |
| Other names
Salsonin, Monosodium salicylate, Sodium o-hydroxybenzoate, Salicylic acid sodium salt, Monosodium 2-hydroxybenzoate, Diuratin
| |
| Identifiers | |
3D model (
JSmol)
|
|
| ChEMBL | |
| ChemSpider | |
| DrugBank | |
| ECHA InfoCard | 100.000.181 |
| EC Number |
|
| KEGG | |
PubChem
CID
|
|
| RTECS number |
|
| UNII | |
CompTox Dashboard (
EPA)
|
|
| |
| |
| Properties | |
| C7H5NaO3 | |
| Molar mass | 160.104 g/mol |
| Appearance | White crystals |
| Melting point | 200 °C (392 °F; 473 K) |
| 25.08 g/100 g (-1.5 °C) 107.9 g/100 g (15 °C) 124.6 g/100 g (25 °C) 141.8 g/100 g (78.5 °C) 179 g/100 g (114 °C) [1] | |
| Solubility | Soluble in glycerol, 1,4-Dioxane, alcohol [1] |
| Solubility in methanol | 26.28 g/100 g (15 °C) 34.73 g/100 g (67.2 °C) [1] |
| Pharmacology | |
| N02BA04 ( WHO) | |
| Hazards | |
| Occupational safety and health (OHS/OSH): | |
Main hazards
|
Harmful |
Eye hazards
|
Irritant |
| GHS labelling: [3] | |

| |
| Warning | |
| H314, H331, H400 | |
| P210, P261, P273, P280, P305+P351+P338, P310 | |
| NFPA 704 (fire diamond) | |
| 250 °C (482 °F; 523 K) | |
| Lethal dose or concentration (LD, LC): | |
LD50 (
median dose)
|
930 mg/kg (rats, oral) [2] |
Except where otherwise noted, data are given for materials in their
standard state (at 25 °C [77 °F], 100 kPa).
| |
Sodium salicylate is a sodium salt of salicylic acid. It can be prepared from sodium phenolate and carbon dioxide under higher temperature and pressure. Historically, it has been synthesized by refluxing methyl salicylate ( wintergreen oil) with an excess of sodium hydroxide. [4]
Properties
Sodium salicylate is of the salicylate family.
Uses
It is used in medicine as an analgesic and antipyretic. Sodium salicylate also acts as non-steroidal anti-inflammatory drug (NSAID), and induces apoptosis in cancer cells [5] [6] [7] and also necrosis. [8] It is also a potential replacement for aspirin for people sensitive to it. It may also be used as a phosphor for the detection of vacuum ultraviolet radiation and electrons. [9]
References
- ^ a b c "sodium salicylate". chemister.ru. Retrieved 8 April 2018.
- ^ Chambers, Michael. "ChemIDplus - 54-21-7 - ABBQHOQBGMUPJH-UHFFFAOYSA-M - Sodium salicylate [USP:JAN] - Similar structures search, synonyms, formulas, resource links, and other chemical information". chem.sis.nlm.nih.gov. Retrieved 8 April 2018.
- ^ Sigma-Aldrich Co., Sodium salicylate. Retrieved on 2014-05-26.
- ^ Lehman, J.W., Operational Organich Chemistry, 4th ed., New Jersey, Prentice Hall, 2009
- ^ Klampfer, Lidija; Jörg Cammenga; Hans-Georg Wisniewski; Stephen D. Nimer (1999-04-01). "Sodium Salicylate Activates Caspases and Induces Apoptosis of Myeloid Leukemia Cell Lines". Blood. 93 (7): 2386–94. doi: 10.1182/blood.V93.7.2386. PMID 10090950.
- ^ Rae, Colin; Susana Langa; Steven J. Tucker; David J. MacEwan (2007-07-31). "Elevated NF-κB responses and FLIP levels in leukemic but not normal lymphocytes: reduction by salicylate allows TNF-induced apoptosis". Proceedings of the National Academy of Sciences of the USA. 104 (31): 12790–5. Bibcode: 2007PNAS..10412790R. doi: 10.1073/pnas.0701437104. PMC 1937545. PMID 17646662.
- ^ Stark, Lesley A.; et al. (May 2007). "Aspirin activates the NF-κB signalling pathway and induces apoptosis in intestinal neoplasia in two in vivo models of human colorectal cancer". Carcinogenesis. 28 (5): 968–76. doi: 10.1093/carcin/bgl220. PMID 17132819.
- ^ Schwenger, Paul; Edward Y. Skolnik; Jan Vilcek (1996-04-05). "Inhibition of Tumor Necrosis Factor-induced p42/p44 Mitogen-Activated Protein Kinase Activation by Sodium Salicylate". The Journal of Biological Chemistry. 271 (14): 8089–94. doi: 10.1074/jbc.271.14.8089. PMID 8626494.
- ^ Samson, James. "Vacuum Ultraviolet Spectroscopy" (PDF). Pied Publications. Archived from the original (PDF) on October 16, 2006. Retrieved July 26, 2012.
External links
Wikimedia Commons has media related to
Sodium salicylate.
