| Neuropilin | |
|---|---|
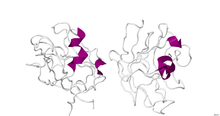
Crystallographic structure of the dimeric B1 domain of human neuropilin 1.
[1] | |
| Identifiers | |
| Symbol | NRP |
| InterPro | IPR014648 |
| Membranome | 16 |
| neuropilin 1 | |||||||
|---|---|---|---|---|---|---|---|
| Identifiers | |||||||
| Symbol | NRP1 | ||||||
| NCBI gene | 8829 | ||||||
| HGNC | 8004 | ||||||
| OMIM | 602069 | ||||||
| PDB | 3I97 | ||||||
| RefSeq | NM_001024628 | ||||||
| UniProt | O14786 | ||||||
| Other data | |||||||
| Locus | Chr. 10 p12 | ||||||
| |||||||
| neuropilin 2 | |||||||
|---|---|---|---|---|---|---|---|
| Identifiers | |||||||
| Symbol | NRP2 | ||||||
| NCBI gene | 8828 | ||||||
| HGNC | 8005 | ||||||
| OMIM | 602070 | ||||||
| RefSeq | NM_201279 | ||||||
| UniProt | O60462 | ||||||
| Other data | |||||||
| Locus | Chr. 2 q34 | ||||||
| |||||||
Neuropilin is a protein receptor active in neurons.
There are two forms of Neuropilins, NRP-1 and NRP-2. Neuropilins are transmembrane glycoproteins, first documented to regulate neurogenesis and angiogenesis by complexing with Plexin receptors/class-3 semaphorin ligands and Vascular Endothelial Growth Factor (VEGF) receptors/VEGF ligands, respectively. [2] [3] Neuropilins predominantly act as co-receptors as they have a very small cytoplasmic domain and thus rely upon other cell surface receptors to transduce their signals across a cell membrane. [2] [3] Recent studies have shown that Neuropilins are multifunctional and can partner with a wide variety of transmembrane receptors. Neuropilins are therefore associated with numerous signalling pathways including those activated by Epidermal Growth Factor (EGF), Fibroblast Growth Factor (FGF), Hepatocyte Growth Factor (HGF), Insulin-like Growth Factor (IGF), Platelet Derived Growth Factor (PDGF) and Transforming Growth Factor beta (TGFβ). [4] [5] Although Neuropilins are commonly found at the cell surface, they have also been reported within the mitochondria and nucleus. [6] [7] Both Neuropilin family members can also be found in soluble forms created by alternative splicing or by ectodomain shedding from the cell surface. [8] [9]
The pleiotropic nature of the NRP receptors results in their involvement in cellular processes, such as axon guidance and angiogenesis, the immune response and remyelination. [10] Therefore, dysregulation of NRP activity has been implicated in many pathological conditions, including many types of cancer and cardiovascular disease. [11] [12] [13] [14]
Applications
Neuropilin-1 is a therapeutic target protein in the treatment for leukemia and lymphoma, since It has been shown that there is increased expression in neuropilin-1 in leukemia and lymphoma cell lines. [15] Also, antagonism of neuropilin-1 has been found to inhibit tumour cell migration and adhesion. [16]
Structure
Neuropilins contain the following four domains:
- N-terminal CUB domain (for complement C1r/C1s, Uegf, Bmp1)
- Coagulation factor 5/8 type, C-terminal ( discoidin domain)
- MAM domain (for meprin, A-5 protein, and receptor protein-tyrosine phosphatase mu)
- C-terminal neuropilin
The structure of B1 domain (coagulation factor 5/8 type) of neuropilin-1 was determined through X-Ray Diffraction with a resolution of 2.90 Å. The secondary structure of this domain is 5% alpha helical and 46% beta sheet. [1]
Ramachandran plot. [17]
References
- ^ a b PDB: 3I97; Jarvis A, Allerston CK, Jia H, Herzog B, Garza-Garcia A, Winfield N, et al. (March 2010). "Small molecule inhibitors of the neuropilin-1 vascular endothelial growth factor A (VEGF-A) interaction". Journal of Medicinal Chemistry. 53 (5): 2215–26. doi: 10.1021/jm901755g. PMC 2841442. PMID 20151671.
- ^ a b Pellet-Many C, Frankel P, Jia H, Zachary I (April 2008). "Neuropilins: structure, function and role in disease". The Biochemical Journal. 411 (2): 211–26. doi: 10.1042/bj20071639. PMID 18363553.
- ^ a b Schwarz Q, Ruhrberg C (January 2010). "Neuropilin, you gotta let me know: should I stay or should I go?". Cell Adhesion & Migration. 4 (1): 61–6. doi: 10.4161/cam.4.1.10207. PMC 2852559. PMID 20026901.
- ^ Kofler N, Simons M (May 2016). "The expanding role of neuropilin: regulation of transforming growth factor-β and platelet-derived growth factor signaling in the vasculature". Current Opinion in Hematology. 23 (3): 260–7. doi: 10.1097/moh.0000000000000233. PMC 4957701. PMID 26849476.
- ^ Roy S, Pramanik A, Chakraborti T, Chakraborti S (2017). "Multifaceted Role of Matrix Metalloproteases on Human Diseases". Proteases in Human Diseases. Springer Singapore. pp. 21–40. doi: 10.1007/978-981-10-3162-5_2. ISBN 978-981-10-3161-8.
- ^ Issitt T, Bosseboeuf E, De Winter N, Dufton N, Gestri G, Senatore V, et al. (January 2019). "Neuropilin-1 Controls Endothelial Homeostasis by Regulating Mitochondrial Function and Iron-Dependent Oxidative Stress". iScience. 11: 205–223. Bibcode: 2019iSci...11..205I. doi: 10.1016/j.isci.2018.12.005. PMC 6327076. PMID 30623799.
- ^ Mehta V, Fields L, Evans IM, Yamaji M, Pellet-Many C, Jones T, et al. (August 2018). "VEGF (Vascular Endothelial Growth Factor) Induces NRP1 (Neuropilin-1) Cleavage via ADAMs (a Disintegrin and Metalloproteinase) 9 and 10 to Generate Novel Carboxy-Terminal NRP1 Fragments That Regulate Angiogenic Signaling". Arteriosclerosis, Thrombosis, and Vascular Biology. 38 (8): 1845–1858. doi: 10.1161/ATVBAHA.118.311118. PMC 6092111. PMID 29880492.
- ^ Rossignol M, Gagnon ML, Klagsbrun M (December 2000). "Genomic organization of human neuropilin-1 and neuropilin-2 genes: identification and distribution of splice variants and soluble isoforms". Genomics. 70 (2): 211–22. doi: 10.1006/geno.2000.6381. PMID 11112349.
- ^ Werneburg S, Buettner FF, Erben L, Mathews M, Neumann H, Mühlenhoff M, et al. (August 2016). "Polysialylation and lipopolysaccharide-induced shedding of E-selectin ligand-1 and neuropilin-2 by microglia and THP-1 macrophages". Glia. 64 (8): 1314–30. doi: 10.1002/glia.23004. PMID 27159043. S2CID 3713077.
- ^ Mecollari V, Nieuwenhuis B, Verhaagen J (2014). "A perspective on the role of class III semaphorin signaling in central nervous system trauma". Frontiers in Cellular Neuroscience. 8: 328. doi: 10.3389/fncel.2014.00328. PMC 4209881. PMID 25386118.
- ^ Niland S, Eble JA (February 2019). "Neuropilins in the Context of Tumor Vasculature". International Journal of Molecular Sciences. 20 (3): 639. doi: 10.3390/ijms20030639. PMC 6387129. PMID 30717262.
- ^ Kofler N, Simons M (May 2016). "The expanding role of neuropilin: regulation of transforming growth factor-β and platelet-derived growth factor signaling in the vasculature". Current Opinion in Hematology. 23 (3): 260–7. doi: 10.1097/MOH.0000000000000233. PMC 4957701. PMID 26849476.
- ^ Pellet-Many C, Mehta V, Fields L, Mahmoud M, Lowe V, Evans I, et al. (November 2015). "Neuropilins 1 and 2 mediate neointimal hyperplasia and re-endothelialization following arterial injury". Cardiovascular Research. 108 (2). Oxford University Press: 288–98. doi: 10.1093/cvr/cvv229. OCLC 927518632. PMC 4614691. PMID 26410366.
- ^ Harman JL, Sayers J, Chapman C, Pellet-Many C (2020-07-21). "Emerging Roles for Neuropilin-2 in Cardiovascular Disease". International Journal of Molecular Sciences. 21 (14): 5154. doi: 10.3390/ijms21145154. PMC 7404143. PMID 32708258.
- ^ Karjalainen K, Jaalouk DE, Bueso-Ramos CE, Zurita AJ, Kuniyasu A, Eckhardt BL, et al. (January 2011). "Targeting neuropilin-1 in human leukemia and lymphoma". Blood. 117 (3): 920–7. doi: 10.1182/blood-2010-05-282921. PMC 3298438. PMID 21063027.
- ^ Jia H, Cheng L, Tickner M, Bagherzadeh A, Selwood D, Zachary I (February 2010). "Neuropilin-1 antagonism in human carcinoma cells inhibits migration and enhances chemosensitivity". British Journal of Cancer. 102 (3): 541–52. doi: 10.1038/sj.bjc.6605539. PMC 2822953. PMID 20087344.
- ^ "MolProbity Ramachandran analysis of PDB structure 3I97" (PDF). www.pdb.org. Archived from the original (PDF) on 2012-09-23. Retrieved 2010-11-21.
External links
- Neuropilins at the U.S. National Library of Medicine Medical Subject Headings (MeSH)