| Dynamin family | |||||||||
|---|---|---|---|---|---|---|---|---|---|
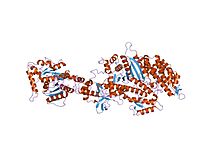 Structure of the nucleotide-free myosin II motor domain from Dictyostelium discoideum fused to the GTPase domain of dynamin I from Rattus norvegicus | |||||||||
| Identifiers | |||||||||
| Symbol | Dynamin_N | ||||||||
| Pfam | PF00350 | ||||||||
| Pfam clan | CL0023 | ||||||||
| InterPro | IPR001401 | ||||||||
| PROSITE | PDOC00362 | ||||||||
| |||||||||
| Dynamin central region | |||||||||
|---|---|---|---|---|---|---|---|---|---|
 Structure of the nucleotide-free myosin II motor domain from Dictyostelium discoideum fused to the GTPase domain of dynamin I from Rattus norvegicus | |||||||||
| Identifiers | |||||||||
| Symbol | Dynamin_M | ||||||||
| Pfam | PF01031 | ||||||||
| InterPro | IPR000375 | ||||||||
| |||||||||
Dynamin is a GTPase responsible for endocytosis in the eukaryotic cell. Dynamin is part of the " dynamin superfamily", which includes classical dynamins, dynamin-like proteins, Mx proteins, OPA1, mitofusins, and GBPs. Members of the dynamin family are principally involved in the scission of newly formed vesicles from the membrane of one cellular compartment and their targeting to, and fusion with, another compartment, both at the cell surface (particularly caveolae internalization) as well as at the Golgi apparatus. [1] [2] [3] Dynamin family members also play a role in many processes including division of organelles, [4] cytokinesis and microbial pathogen resistance.
Structure
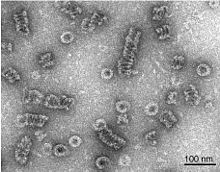
Dynamin itself is a 96 kDa enzyme, and was first isolated when researchers were attempting to isolate new microtubule-based motors from the bovine brain. Dynamin has been extensively studied in the context of clathrin-coated vesicle budding from the cell membrane. [3] [6] Beginning from the N-terminus, Dynamin consists of a GTPase domain connected to a helical stalk domain via a flexible neck region containing a Bundle Signalling Element and GTPase Effector Domain. At the opposite end of the stalk domain is a loop that links to a membrane-binding Pleckstrin homology domain. The protein strand then loops back towards the GTPase domain and terminates with a Proline Rich Domain that binds to the Src Homology domains of many proteins.
Function
During clathrin-mediated endocytosis, the cell membrane invaginates to form a budding vesicle. Dynamin binds to and assembles around the neck of the endocytic vesicle, forming a helical polymer arranged such that the GTPase domains dimerize in an asymmetric manner across helical rungs. [7] [8] The polymer constricts the underlying membrane upon GTP binding and hydrolysis via conformational changes emanating from the flexible neck region that alters the overall helical symmetry. [8] Constriction around the vesicle neck leads to the formation of a hemi-fission membrane state that ultimately results in membrane scission. [2] [6] [9] Constriction may be in part the result of the twisting activity of dynamin, which makes dynamin the only molecular motor known to have a twisting activity. [10]
Types
In mammals, three different dynamin genes have been identified with key sequence differences in their Pleckstrin homology domains leading to differences in the recognition of lipid membranes:
- Dynamin I is expressed in neurons and neuroendocrine cells
- Dynamin II is expressed in most cell types
- Dynamin III is strongly expressed in the testis, but is also present in heart, brain, and lung tissue. [1] [6]
Pharmacology
Small molecule inhibitors of dynamin activity have been developed, including Dynasore [11] [12] and photoswitchable derivatives (Dynazo) [13] for spatiotemporal control of endocytosis with light ( photopharmacology).
Disease implications
Mutations in Dynamin II have been found to cause dominant intermediate Charcot-Marie-Tooth disease. [14] Epileptic encephalopathy–causing de novo mutations in dynamin have been suggested to cause dysfunction of vesicle scission during synaptic vesicle endocytosis. [15]
References
- ^ a b Henley, John R.; Cao, Hong; McNiven, Mark A. (December 16, 1999). "Participation of dynamin in the biogenesis of cytoplasmic vesicles". The FASEB Journal. 13 (9002): S243-7. doi: 10.1096/fasebj.13.9002.S243. PMID 10619136. S2CID 24401725.
- ^ a b Hinshaw, J. "Research statement, Jenny E. Hinshaw, Ph.D." Archived 2021-07-15 at the Wayback Machine National Institute of Diabetes & Digestive & Kidney Diseases, Laboratory of Cell Biochemistry and Biology. Accessed 19 March 2013.
- ^ a b Urrutia R, Henley JR, Cook T, McNiven MA (January 1997). "The dynamins: redundant or distinct functions for an expanding family of related GTPases?". Proceedings of the National Academy of Sciences of the United States of America. 94 (2): 377–384. Bibcode: 1997PNAS...94..377U. doi: 10.1073/pnas.94.2.377. PMC 34135. PMID 9012790.
- ^ Thoms S, Erdmann R (October 2005). "Dynamin-related proteins and Pex11 proteins in peroxisome division and proliferation". The FEBS Journal. 272 (20): 5169–5181. doi: 10.1111/j.1742-4658.2005.04939.x. PMID 16218949.
- ^ Hinshaw JE, Schmid SL (March 1995). "Dynamin self-assembles into rings suggesting a mechanism for coated vesicle budding". Nature. 374 (6518): 190–192. Bibcode: 1995Natur.374..190H. doi: 10.1038/374190a0. PMID 7877694. S2CID 4365628.
- ^ a b c Praefcke GJ, McMahon HT (February 2004). "The dynamin superfamily: universal membrane tubulation and fission molecules?". Nature Reviews. Molecular Cell Biology. 5 (2): 133–147. doi: 10.1038/nrm1313. PMID 15040446. S2CID 6305282.
- ^ Sundborger AC, Fang S, Heymann JA, Ray P, Chappie JS, Hinshaw JE (August 2014). "A dynamin mutant defines a superconstricted prefission state". Cell Reports. 8 (3): 734–742. doi: 10.1016/j.celrep.2014.06.054. PMC 4142656. PMID 25088425.
- ^ a b Kong L, Sochacki KA, Wang H, Fang S, Canagarajah B, Kehr AD, et al. (August 2018). "Cryo-EM of the dynamin polymer assembled on lipid membrane". Nature. 560 (7717): 258–262. Bibcode: 2018Natur.560..258K. doi: 10.1038/s41586-018-0378-6. PMC 6121775. PMID 30069048.
- ^ Mattila JP, Shnyrova AV, Sundborger AC, Hortelano ER, Fuhrmans M, Neumann S, et al. (August 2015). "A hemi-fission intermediate links two mechanistically distinct stages of membrane fission". Nature. 524 (7563): 109–113. Bibcode: 2015Natur.524..109M. doi: 10.1038/nature14509. PMC 4529379. PMID 26123023.
- ^ Roux A, Uyhazi K, Frost A, De Camilli P (May 2006). "GTP-dependent twisting of dynamin implicates constriction and tension in membrane fission". Nature. 441 (7092): 528–531. Bibcode: 2006Natur.441..528R. doi: 10.1038/nature04718. PMID 16648839. S2CID 4413887.
- ^ Macia E, Ehrlich M, Massol R, Boucrot E, Brunner C, Kirchhausen T (June 2006). "Dynasore, a cell-permeable inhibitor of dynamin". Developmental Cell. 10 (6): 839–850. doi: 10.1016/j.devcel.2006.04.002. PMID 16740485.
- ^ Eschenburg S, Reubold TF (November 2018). "Modulation of dynamin function by small molecules". Biological Chemistry. 399 (12): 1421–1432. doi: 10.1515/hsz-2018-0257. PMID 30067507. S2CID 51895475.
- ^ Camarero N, Trapero A, Pérez-Jiménez A, Macia E, Gomila-Juaneda A, Martín-Quirós A, et al. (September 2020). "Correction: Photoswitchable dynasore analogs to control endocytosis with light". Chemical Science. 11 (35): 9712. doi: 10.1039/D0SC90189J. PMC 7495901. PMID 33016959.
- ^ Züchner S, Noureddine M, Kennerson M, Verhoeven K, Claeys K, De Jonghe P, et al. (March 2005). "Mutations in the pleckstrin homology domain of dynamin 2 cause dominant intermediate Charcot-Marie-Tooth disease". Nature Genetics. 37 (3): 289–294. doi: 10.1038/ng1514. PMID 15731758. S2CID 19191581.
- ^ Dhindsa RS, Bradrick SS, Yao X, Heinzen EL, Petrovski S, Krueger BJ, et al. (June 2015). "Epileptic encephalopathy-causing mutations in DNM1 impair synaptic vesicle endocytosis". Neurology. Genetics. 1 (1): e4. doi: 10.1212/01.NXG.0000464295.65736.da. PMC 4821085. PMID 27066543.
External links
- Dynamins at the U.S. National Library of Medicine Medical Subject Headings (MeSH)