 | |
| Clinical data | |
|---|---|
| ATC code |
|
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| ChemSpider | |
| UNII | |
| ChEMBL | |
| CompTox Dashboard ( EPA) | |
| ECHA InfoCard | 100.111.408 |
| Chemical and physical data | |
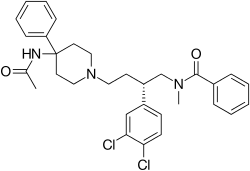
| Formula | C31H35Cl2N3O2 |
| Molar mass | 552.54 g·mol−1 |
| 3D model ( JSmol) | |
| |
| |
| | |
Saredutant (SR-48,968) is a drug that acts as a NK2 receptor antagonist. [1] It was under development by Sanofi-Aventis as a novel antidepressant and anxiolytic and made it to phase III clinical trials. However, in May 2009, Sanofi-Aventis published its quarterly results and announced the cessation of 14 research/development projects, among which was saredutant for the treatment of major depressive disorder. [2]
See also
References
- ^ Hopkins CR (October 2010). "ACS chemical neuroscience molecule spotlight on Saredutant". ACS Chemical Neuroscience. 1 (10): 653–4. doi: 10.1021/cn100061r. PMC 3368631. PMID 22776916.
- ^ "Letter to the stockholders of Sanofi-Aventis" (PDF). May 2009. Archived from the original (PDF) on 21 December 2010.