| RambergâBĂ€cklund reaction | |
|---|---|
| Named after |
Ludwig Ramberg Birger BĂ€cklund |
| Reaction type | Rearrangement reaction |
| Identifiers | |
| Organic Chemistry Portal | ramberg-baecklund-reaction |
| RSC ontology ID | RXNO:0000094 |
The RambergâBĂ€cklund reaction is an organic reaction converting an α-halo sulfone into an alkene in presence of a base with extrusion of sulfur dioxide. [1] The reaction is named after the two Swedish chemists Ludwig Ramberg and Birger BĂ€cklund. The carbanion formed by deprotonation gives an unstable episulfone that decomposes with elimination of sulfur dioxide. This elimination step is considered to be a concerted cheletropic extrusion.[ citation needed]

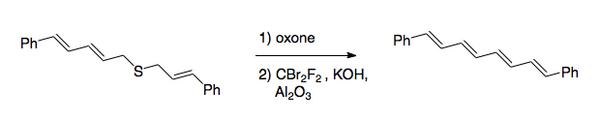
The overall transformation is the conversion of the carbonâsulfur bonds to a carbonâcarbon double bond. The original procedure involved halogenation of a sulfide, followed by oxidation to the sulfone. Recently, the preferred method has reversed the order of the steps. After the oxidation, which is normally done with a peroxy acid, halogenation is done under basic conditions by use of dibromodifluoromethane for the halogen transfer step. [2] This method was used to synthesize 1,8-diphenyl-1,3,5,7-octatetraene.
Applications
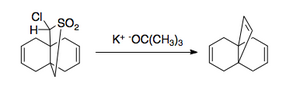
The RambergâBĂ€cklund reaction has several applications. Due to the nature of elimination, it can be applied to both small rings [3],
and large rings containing a double bond [4].
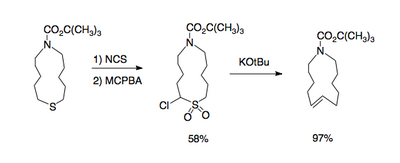
This reaction type gives access to 1,2-dimethylenecyclohexane [5]

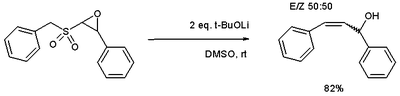
and the epoxide variation [6] access to allyl alcohols.
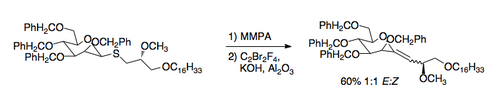
A recently developed application of the RambergâBĂ€cklund reaction is the synthesis of C-glycosides. The required thioethers can be prepared easily by exchange with a thiol. The application of the RambergâBĂ€cklund conditions then leads to an exocyclic vinyl ether that can be reduced to the C-nucleoside [7].
In a variation, oxidation of a sulfamide generates a azo compound. [1]
Substrates
The necessary α-halo sulfones are accessible through oxidation of the corresponding α-halo sulfides with peracids such as meta-chloroperbenzoic acid; oxidation of sulfides takes place selectively in the presence of alkenes and alcohols. α-Halo sulfides may in turn be synthesized through the treatment of sulfides with halogen electrophiles such as N-chlorosuccinimide or N-bromosuccinimide. [8]
Mechanism
The sulfone group contains an acidic proton in one of the α-positions which is abstracted by a strong base (scheme 1). The negative charge placed on this position (formally a carbanion) is transferred to the halogen residing on the other α-position in a nucleophilic displacement temporarily forming a three-membered cyclic sulfone. This intermediate is unstable and releases sulfur dioxide to form the alkene. Mixtures of cis isomer and trans isomer are usually obtained. [9]
The Favorskii rearrangement and the Eschenmoser sulfide contraction are conceptually related reactions.
References
- ^ Ohme, R.; Preuschhof, H.; Heyne, H.-U. (1988). "Azoethane". Organic Syntheses; Collected Volumes, vol. 6, p. 78.
- ^ Ramberg, Ludwig; BĂ€cklund, Birger (1940). "The reactions of some monohalogen derivatives of diethyl sulfone". Archives of Chemistry, Mineralogy and Geology. 27 (13A): 1â50. ISSN 0365-3781.
-
^ Chan, Tze-Lock; Fong, Sun; Li, Yu; Man, Tim-On; Poon, Chi-Duen (1994). "A new one-flask Ramberg–BĂ€cklund reaction".
Journal of the Chemical Society, Chemical Communications (15): 1771â1772.
doi:
10.1039/C39940001771.
Cao, Xiao-Ping (2002). "Stereoselective synthesis of substituted all-trans 1,3,5,7-octatetraenes by a modified Ramberg–BĂ€cklund reaction". Tetrahedron. 58 (7): 1301â1307. doi: 10.1016/S0040-4020(01)01224-8. - ^ Paquette, Leo A.; Philips, J. Christopher; Wingard, Robert E. (1971). "α-Halo sulfones. XVIII. Ramberg–Baecklund rearrangement as a synthetic entry to unsaturated propellanes". Journal of the American Chemical Society. 93 (18): 4516â4522. doi: 10.1021/ja00747a029.
- ^ Magee, D. I.; Beck, E. J. (August 2000). "The use of the Ramberg–BĂ€cklund rearrangement for the formation of aza-macrocycles: a total synthesis of manzamine C". Canadian Journal of Chemistry. 78 (8): 1060â1066. doi: 10.1139/v00-103.
- ^ Böhme, Horst; Gran, Heinz-Joachim (12 July 1952). "Ăber die Einwirkung von Chlor auf ThioĂ€ther und Mercaptale" [On the action of chlorine on thioether and mercaptals]. Justus Liebigs Annalen der Chemie (in German). 577: 68â77. doi: 10.1002/jlac.19525770109.
- ^ Paquette, Leo A. (2005). "The Ramberg–BĂ€cklund Rearrangement". Organic Reactions. Vol. 25. pp. 1â71. doi: 10.1002/0471264180.or025.01. ISBN 9780471264187.
- ^ Block, Eric; Aslam, Mohammad (1987). "A General Synthetic Method for the Preparation of Conjugated Dienes From Olefins Using Bromomethanesulfonyl Bromide: 1,2-Dimethylenecyclohexane". Organic Syntheses. 65: 90. doi: 10.15227/orgsyn.065.0090; Collected Volumes, vol. 8, p. 212.
- ^ Evans, P.; Johnson, P.; Taylor, R. J. K. (April 2006). "The Epoxy-Ramberg–BĂ€cklund Reaction (ERBR): A Sulfone-Based Method for the Synthesis of Allylic Alcohols". European Journal of Organic Chemistry. 2006 (7): 1740â1754. doi: 10.1002/ejoc.200500956.
- ^ Griffin, F. K.; Paterson, D. E.; Murphy, P. V.; Taylor, R. J. K. (August 2002). "ChemInform Abstract: A New Route to exo-Glycals Using the Ramberg–Baecklund Rearrangement". ChemInform. 33 (33): 1305. doi: 10.1002/chin.200233219.