| |
| |

| |
| Names | |
|---|---|
|
IUPAC name
Sulfur dioxide
| |
Other names
| |
| Identifiers | |
3D model (
JSmol)
|
|
| 3535237 | |
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.028.359 |
| EC Number |
|
| E number | E220 (preservatives) |
| 1443 | |
| KEGG | |
| MeSH | Sulfur+dioxide |
PubChem
CID
|
|
| RTECS number |
|
| UNII | |
| UN number | 1079, 2037 |
CompTox Dashboard (
EPA)
|
|
| |
| |
| Properties | |
| SO 2 | |
| Molar mass | 64.066 g/mol |
| Appearance | Colorless and pungent gas |
| Odor | Pungent; similar to a just-struck match [1] |
| Density | 2.619 kg m−3 [2] |
| Melting point | −72 °C; −98 °F; 201 K |
| Boiling point | −10 °C (14 °F; 263 K) |
| 94 g/L
[3] forms sulfurous acid | |
| Vapor pressure | 230 kPa at 10 °C; 330 kPa at 20 °C; 462 kPa at 30 °C; 630 kPa at 40 °C [4] |
| Acidity (pKa) | ~1.81 |
| Basicity (pKb) | ~12.19 |
| −18.2·10−6 cm3/mol | |
| Viscosity | 12.82 μPa·s [5] |
| Structure | |
| C2v | |
| Digonal | |
| Dihedral | |
| 1.62 D | |
| Thermochemistry | |
Std molar
entropy (S⦵298) |
248.223 J K−1 mol−1 |
Std enthalpy of
formation (ΔfH⦵298) |
−296.81 kJ mol−1 |
| Hazards | |
| GHS labelling: | |


| |
| Danger | |
| H314, H331 [6] | |
| NFPA 704 (fire diamond) | |
| Lethal dose or concentration (LD, LC): | |
LC50 (
median concentration)
|
3000 ppm (mouse, 30 min) 2520 ppm (rat, 1 hr) [8] |
LCLo (
lowest published)
|
993 ppm (rat, 20 min) 611 ppm (rat, 5 hr) 764 ppm (mouse, 20 min) 1000 ppm (human, 10 min) 3000 ppm (human, 5 min) [8] |
| NIOSH (US health exposure limits): | |
PEL (Permissible)
|
TWA 5 ppm (13 mg/m3) [7] |
REL (Recommended)
|
TWA 2 ppm (5 mg/m3) ST 5 ppm (13 mg/m3) [7] |
IDLH (Immediate danger)
|
100 ppm [7] |
| Related compounds | |
|
Sulfur monoxide Sulfur trioxide Disulfur monoxide | |
Related compounds
|
Ozone
Selenium dioxide |
Except where otherwise noted, data are given for materials in their
standard state (at 25 °C [77 °F], 100 kPa).
| |
Sulfur dioxide (
IUPAC-recommended spelling) or sulphur dioxide (traditional
Commonwealth English) is the
chemical compound with the formula
S
O
2. It is a colorless
gas with a pungent smell that is responsible for the odor of burnt
matches. It is released naturally by
volcanic activity and is produced as a by-product of
copper extraction and the burning of
sulfur-
bearing fossil fuels.
[9]
Sulfur dioxide is somewhat toxic to humans, although only when inhaled in relatively large quantities for a period of several minutes or more. It was known to medieval alchemists as "volatile spirit of sulfur". [10]
Structure and bonding
SO2 is a bent molecule with C2v symmetry point group. A valence bond theory approach considering just s and p orbitals would describe the bonding in terms of resonance between two resonance structures.

The sulfur–oxygen bond has a bond order of 1.5. There is support for this simple approach that does not invoke d orbital participation. [11] In terms of electron-counting formalism, the sulfur atom has an oxidation state of +4 and a formal charge of +1.
Occurrence

Sulfur dioxide is found on Earth and exists in very small concentrations in the atmosphere at about 15 ppb. [12]
On other planets, sulfur dioxide can be found in various concentrations, the most significant being the atmosphere of Venus, where it is the third-most abundant atmospheric gas at 150 ppm. There, it reacts with water to form clouds of Sulfurous acid (SO2 + H2O ⇌ HSO−3+ H+) , and is a key component of the planet's global atmospheric sulfur cycle and contributes to global warming. [13] It has been implicated as a key agent in the warming of early Mars, with estimates of concentrations in the lower atmosphere as high as 100 ppm, [14] though it only exists in trace amounts. On both Venus and Mars, as on Earth, its primary source is thought to be volcanic. The atmosphere of Io, a natural satellite of Jupiter, is 90% sulfur dioxide [15] and trace amounts are thought to also exist in the atmosphere of Jupiter. The James Webb Space Telescope has observed the presence of sulfur dioxide on the exoplanet WASP-39b, where it is formed through photochemistry in the planet's atmosphere. [16]
As an ice, it is thought to exist in abundance on the Galilean moons—as subliming ice or frost on the trailing hemisphere of Io, [17] and in the crust and mantle of Europa, Ganymede, and Callisto, possibly also in liquid form and readily reacting with water. [18]
Production
Sulfur dioxide is primarily produced for sulfuric acid manufacture (see contact process, but other processes predated that at least since 16th century [10]). In the United States in 1979, 23.6 million metric tons (26 million U.S. short tons) of sulfur dioxide were used in this way, compared with 150,000 metric tons (165,347 U.S. short tons) used for other purposes. Most sulfur dioxide is produced by the combustion of elemental sulfur. Some sulfur dioxide is also produced by roasting pyrite and other sulfide ores in air. [19]
Combustion routes
Sulfur dioxide is the product of the burning of sulfur or of burning materials that contain sulfur:
- 1⁄8 S8 + O2 → SO2, ΔH = −297 kJ/mol
To aid combustion, liquified sulfur (140–150 °C, 284-302 °F) is sprayed through an atomizing nozzle to generate fine drops of sulfur with a large surface area. The reaction is exothermic, and the combustion produces temperatures of 1000–1600 °C (1832–2912 °F). The significant amount of heat produced is recovered by steam generation that can subsequently be converted to electricity. [19]
The combustion of hydrogen sulfide and organosulfur compounds proceeds similarly. For example:
- H2S + 3⁄2 O2 → SO2 + H2O
The roasting of sulfide ores such as pyrite, sphalerite, and cinnabar (mercury sulfide) also releases SO2: [20]
- 2 FeS2 + 11⁄2 O2 → Fe2O3 + 4 SO2
- ZnS + 3⁄2 O2 → ZnO + SO2
- HgS + O2 → Hg + SO2
- 2 FeS + 7⁄2 O2 → Fe2O3 + 2 SO2
A combination of these reactions is responsible for the largest source of sulfur dioxide, volcanic eruptions. These events can release millions of tons of SO2.
Reduction of higher oxides
Sulfur dioxide can also be a byproduct in the manufacture of calcium silicate cement; CaSO4 is heated with coke and sand in this process:
- 2 CaSO4 + 2 SiO2 + C → 2 CaSiO3 + 2 SO2 + CO2
Until the 1970s, commercial quantities of sulfuric acid and cement were produced by this process in Whitehaven, England. Upon being mixed with shale or marl, and roasted, the sulfate liberated sulfur dioxide gas, used in sulfuric acid production, the reaction also produced calcium silicate, a precursor in cement production. [21]
On a laboratory scale, the action of hot concentrated sulfuric acid on copper turnings produces sulfur dioxide.
- Cu + 2 H2SO4 → CuSO4 + SO2 + 2 H2O
Tin also reacts with concentrated sulfuric acid but it produces tin(II) sulfate which can later be pyrolyzed at 360°C into tin dioxide and dry sulfur dioxide.
- Sn + H2SO4 → SnSO4 + H2
- SnSO4 → SnO2 + SO2
From sulfites
The reverse reaction occurs upon acidification:
- H+ + HSO−3 → SO2 + H2O
Reactions
Sulfites results by the action of aqueous base on sulfur dioxide:
- SO2 + 2 NaOH → Na2SO3 + H2O
Sulfur dioxide is a mild but useful reducing agent. It is oxidized by halogens to give the sulfuryl halides, such as sulfuryl chloride:
- SO2 + Cl2 → SO2Cl2
Sulfur dioxide is the oxidising agent in the Claus process, which is conducted on a large scale in oil refineries. Here, sulfur dioxide is reduced by hydrogen sulfide to give elemental sulfur:
- SO2 + 2 H2S → 3 S + 2 H2O
The sequential oxidation of sulfur dioxide followed by its hydration is used in the production of sulfuric acid.
- SO2 + H2O + 1⁄2 O2 → H2SO4
Sulfur dioxide dissolves in water to give " sulfurous acid", which cannot be isolated and is instead an acidic solution of bisulfite, and possibly sulfite, ions.
- SO2 + H2O ⇌ HSO−3 + H+ Ka = 1.54×10−2; pKa = 1.81
Laboratory reactions
Sulfur dioxide is one of the few common acidic yet reducing gases. It turns moist litmus pink (being acidic), then white (due to its bleaching effect). It may be identified by bubbling it through a dichromate solution, turning the solution from orange to green (Cr3+ (aq)). It can also reduce ferric ions to ferrous. [22]
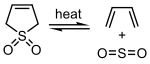
Sulfur dioxide can react with certain 1,3- dienes in a cheletropic reaction to form cyclic sulfones. This reaction is exploited on an industrial scale for the synthesis of sulfolane, which is an important solvent in the petrochemical industry.
Sulfur dioxide can bind to metal ions as a ligand to form metal sulfur dioxide complexes, typically where the transition metal is in oxidation state 0 or +1. Many different bonding modes (geometries) are recognized, but in most cases, the ligand is monodentate, attached to the metal through sulfur, which can be either planar and pyramidal η1. [9] As a η1-SO2 (S-bonded planar) ligand sulfur dioxide functions as a Lewis base using the lone pair on S. SO2 functions as a Lewis acids in its η1-SO2 (S-bonded pyramidal) bonding mode with metals and in its 1:1 adducts with Lewis bases such as dimethylacetamide and trimethyl amine. When bonding to Lewis bases the acid parameters of SO2 are EA = 0.51 and EA = 1.56.
Uses
The overarching, dominant use of sulfur dioxide is in the production of sulfuric acid. [19]
Precursor to sulfuric acid
Sulfur dioxide is an intermediate in the production of sulfuric acid, being converted to sulfur trioxide, and then to oleum, which is made into sulfuric acid. Sulfur dioxide for this purpose is made when sulfur combines with oxygen. The method of converting sulfur dioxide to sulfuric acid is called the contact process. Several million tons are produced annually for this purpose.
Food preservative
Sulfur dioxide is sometimes used as a preservative for dried apricots, dried figs, and other dried fruits, owing to its antimicrobial properties and ability to prevent oxidation, [23] and is called E220 [24] when used in this way in Europe. As a preservative, it maintains the colorful appearance of the fruit and prevents rotting. Historically, molasses was "sulfured" as a preservative and also to lighten its color. Treatment of dried fruit was usually done outdoors, by igniting sublimed sulfur and burning in an enclosed space with the fruits. [25] Fruits may be sulfured by dipping them into an either sodium bisulfite, sodium sulfite or sodium metabisulfite. [25]
Winemaking
Sulfur dioxide was first used in winemaking by the Romans, when they discovered that burning sulfur candles inside empty wine vessels keeps them fresh and free from vinegar smell. [26]
It is still an important compound in winemaking, and is measured in parts per million (ppm) in wine. It is present even in so-called unsulfurated wine at concentrations of up to 10 mg/L. [27] It serves as an antibiotic and antioxidant, protecting wine from spoilage by bacteria and oxidation - a phenomenon that leads to the browning of the wine and a loss of cultivar specific flavors. [28] [29] Its antimicrobial action also helps minimize volatile acidity. Wines containing sulfur dioxide are typically labeled with "containing sulfites".
Sulfur dioxide exists in wine in free and bound forms, and the combinations are referred to as total SO2. Binding, for instance to the carbonyl group of acetaldehyde, varies with the wine in question. The free form exists in equilibrium between molecular SO2 (as a dissolved gas) and bisulfite ion, which is in turn in equilibrium with sulfite ion. These equilibria depend on the pH of the wine. Lower pH shifts the equilibrium towards molecular (gaseous) SO2, which is the active form, while at higher pH more SO2 is found in the inactive sulfite and bisulfite forms. The molecular SO2 is active as an antimicrobial and antioxidant, and this is also the form which may be perceived as a pungent odor at high levels. Wines with total SO2 concentrations below 10 ppm do not require "contains sulfites" on the label by US and EU laws. The upper limit of total SO2 allowed in wine in the US is 350 ppm; in the EU it is 160 ppm for red wines and 210 ppm for white and rosé wines. In low concentrations, SO2 is mostly undetectable in wine, but at free SO2 concentrations over 50 ppm, SO2 becomes evident in the smell and taste of wine.[ citation needed]
SO2 is also a very important compound in winery sanitation. Wineries and equipment must be kept clean, and because bleach cannot be used in a winery due to the risk of cork taint, [30] a mixture of SO2, water, and citric acid is commonly used to clean and sanitize equipment. Ozone (O3) is now used extensively for sanitizing in wineries due to its efficacy, and because it does not affect the wine or most equipment. [31]
As a reducing agent
Sulfur dioxide is also a good reductant. In the presence of water, sulfur dioxide is able to decolorize substances. Specifically, it is a useful reducing bleach for papers and delicate materials such as clothes. This bleaching effect normally does not last very long. Oxygen in the atmosphere reoxidizes the reduced dyes, restoring the color. In municipal wastewater treatment, sulfur dioxide is used to treat chlorinated wastewater prior to release. Sulfur dioxide reduces free and combined chlorine to chloride. [32]
Sulfur dioxide is fairly soluble in water, and by both IR and Raman spectroscopy; the hypothetical sulfurous acid, H2SO3, is not present to any extent. However, such solutions do show spectra of the hydrogen sulfite ion, HSO3−, by reaction with water, and it is in fact the actual reducing agent present:
- SO2 + H2O ⇌ HSO3− + H+
As a fumigant
In the beginning of the 20th century, sulfur dioxide was used in Buenos Aires as a fumigant to kill rats that carried the Yersinia pestis bacterium, which causes bubonic plague. The application was successful, and the application of this method was extended to other areas in South America. In Buenos Aires, where these apparatuses were known as Sulfurozador, but later also in Rio de Janeiro, New Orleans and San Francisco, the sulfur dioxide treatment machines were brought into the streets to enable extensive disinfection campaigns, with effective results. [33]
Biochemical and biomedical roles
Sulfur dioxide or its conjugate base bisulfite is produced biologically as an intermediate in both sulfate-reducing organisms and in sulfur-oxidizing bacteria, as well. The role of sulfur dioxide in mammalian biology is not yet well understood. [34] Sulfur dioxide blocks nerve signals from the pulmonary stretch receptors and abolishes the Hering–Breuer inflation reflex.
It is considered that endogenous sulfur dioxide plays a significant physiological role in regulating cardiac and blood vessel function, and aberrant or deficient sulfur dioxide metabolism can contribute to several different cardiovascular diseases, such as arterial hypertension, atherosclerosis, pulmonary arterial hypertension, and stenocardia. [35]
It was shown that in children with pulmonary arterial hypertension due to congenital heart diseases the level of homocysteine is higher and the level of endogenous sulfur dioxide is lower than in normal control children. Moreover, these biochemical parameters strongly correlated to the severity of pulmonary arterial hypertension. Authors considered homocysteine to be one of useful biochemical markers of disease severity and sulfur dioxide metabolism to be one of potential therapeutic targets in those patients. [36]
Endogenous sulfur dioxide also has been shown to lower the proliferation rate of endothelial smooth muscle cells in blood vessels, via lowering the MAPK activity and activating adenylyl cyclase and protein kinase A. [37] Smooth muscle cell proliferation is one of important mechanisms of hypertensive remodeling of blood vessels and their stenosis, so it is an important pathogenetic mechanism in arterial hypertension and atherosclerosis.
Endogenous sulfur dioxide in low concentrations causes endothelium-dependent vasodilation. In higher concentrations it causes endothelium-independent vasodilation and has a negative inotropic effect on cardiac output function, thus effectively lowering blood pressure and myocardial oxygen consumption. The vasodilating and bronchodilating effects of sulfur dioxide are mediated via ATP-dependent calcium channels and L-type ("dihydropyridine") calcium channels. Endogenous sulfur dioxide is also a potent antiinflammatory, antioxidant and cytoprotective agent. It lowers blood pressure and slows hypertensive remodeling of blood vessels, especially thickening of their intima. It also regulates lipid metabolism. [38]
Endogenous sulfur dioxide also diminishes myocardial damage, caused by isoproterenol adrenergic hyperstimulation, and strengthens the myocardial antioxidant defense reserve. [39]
As a reagent and solvent in the laboratory
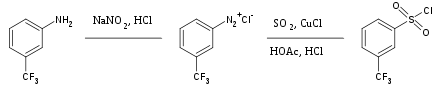
Sulfur dioxide is a versatile inert solvent widely used for dissolving highly oxidizing salts. It is also used occasionally as a source of the sulfonyl group in organic synthesis. Treatment of aryl diazonium salts with sulfur dioxide and cuprous chloride yields the corresponding aryl sulfonyl chloride, for example: [40]
As a result of its very low Lewis basicity, it is often used as a low-temperature solvent/diluent for superacids like magic acid (FSO3H/SbF5), allowing for highly reactive species like tert-butyl cation to be observed spectroscopically at low temperature (though tertiary carbocations do react with SO2 above about –30 °C, and even less reactive solvents like SO2ClF must be used at these higher temperatures). [41]
As a refrigerant
Being easily condensed and possessing a high heat of evaporation, sulfur dioxide is a candidate material for refrigerants. Before the development of chlorofluorocarbons, sulfur dioxide was used as a refrigerant in home refrigerators.
Safety

Ingestion
In the United States, the Center for Science in the Public Interest lists the two food preservatives, sulfur dioxide and sodium bisulfite, as being safe for human consumption except for certain asthmatic individuals who may be sensitive to them, especially in large amounts. [42] Symptoms of sensitivity to sulfiting agents, including sulfur dioxide, manifest as potentially life-threatening trouble breathing within minutes of ingestion. [43] Sulphites may also cause symptoms in non-asthmatic individuals, namely dermatitis, urticaria, flushing, hypotension, abdominal pain and diarrhea, and even life-threatening anaphylaxis. [44]
Inhalation
Incidental exposure to sulfur dioxide is routine, e.g. the smoke from matches, coal, and sulfur-containing fuels like bunker fuel. Relative to other chemicals, it is only mildly toxic and requires high concentrations to be actively hazardous. [45] However, its ubiquity makes it a major air pollutant with significant impacts on human health. [46]
In 2008, the American Conference of Governmental Industrial Hygienists reduced the short-term exposure limit to 0.25 parts per million (ppm). In the US, the OSHA set the PEL at 5 ppm (13 mg/m3) time-weighted average. Also in the US, NIOSH set the IDLH at 100 ppm. [47] In 2010, the EPA "revised the primary SO2 NAAQS by establishing a new one-hour standard at a level of 75 parts per billion (ppb). EPA revoked the two existing primary standards because they would not provide additional public health protection given a one-hour standard at 75 ppb." [46]
Environmental role
Air pollution

Major volcanic eruptions have an overwhelming effect on sulfate aerosol concentrations in the years when they occur: eruptions ranking 4 or greater on the Volcanic Explosivity Index inject SO2 and water vapor directly into the stratosphere, where they react to create sulfate aerosol plumes. [48] Volcanic emissions vary significantly in composition, and have complex chemistry due to the presence of ash particulates and a wide variety of other elements in the plume. Only stratovolcanoes containing primarily felsic magmas are responsible for these fluxes, as mafic magma erupted in shield volcanoes doesn't result in plumes which reach the stratosphere. [49] However, before the Industrial Revolution, dimethyl sulfide pathway was the largest contributor to sulfate aerosol concentrations in a more average year with no major volcanic activity. According to the IPCC First Assessment Report, published in 1990, volcanic emissions usually amounted to around 10 million tons in 1980s, while dimethyl sulfide amounted to 40 million tons. Yet, by that point, the global human-caused emissions of sulfur into the atmosphere became "at least as large" as all natural emissions of sulfur-containing compounds combined: they were at less than 3 million tons per year in 1860, and then they increased to 15 million tons in 1900, 40 million tons in 1940 and about 80 millions in 1980. The same report noted that "in the industrialized regions of Europe and North America, anthropogenic emissions dominate over natural emissions by about a factor of ten or even more". [50] In the eastern United States, sulfate particles were estimated to account for 25% or more of all air pollution. [51] Exposure to sulfur dioxide emissions by coal power plants (coal PM2.5) in the US was associated with 2.1 times greater mortality risk than exposure to PM2.5 from all sources. [52] Meanwhile, the Southern Hemisphere had much lower concentrations due to being much less densely populated, with an estimated 90% of the human population in the north. In the early 1990s, anthropogenic sulfur dominated in the Northern Hemisphere, where only 16% of annual sulfur emissions were natural, yet amounted for less than half of the emissions in the Southern Hemisphere. [53]

Such an increase in sulfate aerosol emissions had a variety of effects. At the time, the most visible one was acid rain, caused by precipitation from clouds carrying high concentrations of sulfate aerosols in the troposphere. [54] At its peak, acid rain has eliminated brook trout and some other fish species and insect life from lakes and streams in geographically sensitive areas, such as Adirondack Mountains in the United States. [55] Acid rain worsens soil function as some of its microbiota is lost and heavy metals like aluminium are mobilized (spread more easily) while essential nutrients and minerals such as magnesium can leach away because of the same. Ultimately, plants unable to tolerate lowered pH are killed, with montane forests being some of the worst-affected ecosystems due to their regular exposure to sulfate-carrying fog at high altitudes. [56] [57] [58] [59] [60] While acid rain was too dilute to affect human health directly, breathing smog or even any air with elevated sulfate concentrations is known to contribute to heart and lung conditions, including asthma and bronchitis. [51] Further, this form of pollution is linked to preterm birth and low birth weight, with a study of 74,671 pregnant women in Beijing finding that every additional 100 μg/m3 of SO2 in the air reduced infants' weight by 7.3 g, making it and other forms of air pollution the largest attributable risk factor for low birth weight ever observed. [61]
Control measures

Due largely to the US EPA's Acid Rain Program, the U.S. has had a 33% decrease in emissions between 1983 and 2002 (see table). This improvement resulted in part from flue-gas desulfurization, a technology that enables SO2 to be chemically bound in power plants burning sulfur-containing coal or petroleum.
| Year | SO2 |
|---|---|
| 1970 | 31,161,000 short tons (28.3 Mt) |
| 1980 | 25,905,000 short tons (23.5 Mt) |
| 1990 | 23,678,000 short tons (21.5 Mt) |
| 1996 | 18,859,000 short tons (17.1 Mt) |
| 1997 | 19,363,000 short tons (17.6 Mt) |
| 1998 | 19,491,000 short tons (17.7 Mt) |
| 1999 | 18,867,000 short tons (17.1 Mt) |
In particular, calcium oxide (lime) reacts with sulfur dioxide to form calcium sulfite:
- CaO + SO2 → CaSO3
Aerobic oxidation of the CaSO3 gives CaSO4, anhydrite. Most gypsum sold in Europe comes from flue-gas desulfurization.
To control sulfur emissions, dozens of methods with relatively high efficiencies have been developed for fitting of coal-fired power plants. [63] Sulfur can be removed from coal during burning by using limestone as a bed material in fluidized bed combustion. [64]
Sulfur can also be removed from fuels before burning, preventing formation of SO2 when the fuel is burnt. The Claus process is used in refineries to produce sulfur as a byproduct. The Stretford process has also been used to remove sulfur from fuel. Redox processes using iron oxides can also be used, for example, Lo-Cat [65] or Sulferox. [66]
Fuel additives such as calcium additives and magnesium carboxylate may be used in marine engines to lower the emission of sulfur dioxide gases into the atmosphere. [67]
Impact on climate change
Projected impacts

Since changes in aerosol concentrations already have an impact on the global climate, they would necessarily influence future projections as well. In fact, it is impossible to fully estimate the warming impact of all greenhouse gases without accounting for the counteracting cooling from aerosols. [77] [78]
Regardless of the current strength of aerosol cooling, all future climate change scenarios project decreases in particulates and this includes the scenarios where 1.5 °C (2.7 °F) and 2 °C (3.6 °F) targets are met: their specific emission reduction targets assume the need to make up for lower dimming. [79] Since models estimate that the cooling caused by sulfates is largely equivalent to the warming caused by atmospheric methane (and since methane is a relatively short-lived greenhouse gas), it is believed that simultaneous reductions in both would effectively cancel each other out. [80]
[81] Yet, in the recent years, methane concentrations had been increasing at rates exceeding their previous period of peak growth in the 1980s, [82] [83] with wetland methane emissions driving much of the recent growth, [84] [85] while air pollution is getting cleaned up aggressively. [86] These trends are some of the main reasons why 1.5 °C (2.7 °F) warming is now expected around 2030, as opposed to the mid-2010s estimates where it would not occur until 2040. [77]Solar geoengineering
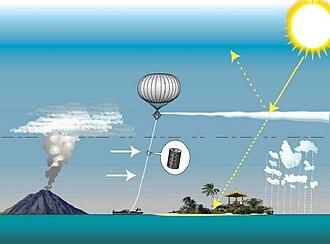
Properties
Table of thermal and physical properties of saturated liquid sulfur dioxide: [98] [99]
| Temperature (°C) | Density (kg/m^3) | Specific heat (kJ/kg K) | Kinematic viscosity (m^2/s) | Conductivity (W/m K) | Thermal diffusivity (m^2/s) | Prandtl Number | Bulk modulus (K^-1) |
| -50 | 1560.84 | 1.3595 | 4.84E-07 | 0.242 | 1.14E-07 | 4.24 | - |
| -40 | 1536.81 | 1.3607 | 4.24E-07 | 0.235 | 1.13E-07 | 3.74 | - |
| -30 | 1520.64 | 1.3616 | 3.71E-07 | 0.23 | 1.12E-07 | 3.31 | - |
| -20 | 1488.6 | 1.3624 | 3.24E-07 | 0.225 | 1.11E-07 | 2.93 | - |
| -10 | 1463.61 | 1.3628 | 2.88E-07 | 0.218 | 1.10E-07 | 2.62 | - |
| 0 | 1438.46 | 1.3636 | 2.57E-07 | 0.211 | 1.08E-07 | 2.38 | - |
| 10 | 1412.51 | 1.3645 | 2.32E-07 | 0.204 | 1.07E-07 | 2.18 | - |
| 20 | 1386.4 | 1.3653 | 2.10E-07 | 0.199 | 1.05E-07 | 2 | 1.94E-03 |
| 30 | 1359.33 | 1.3662 | 1.90E-07 | 0.192 | 1.04E-07 | 1.83 | - |
| 40 | 1329.22 | 1.3674 | 1.73E-07 | 0.185 | 1.02E-07 | 1.7 | - |
| 50 | 1299.1 | 1.3683 | 1.62E-07 | 0.177 | 9.99E-08 | 1.61 | - |
See also
References
- ^ Sulfur dioxide Archived 2019-12-30 at the Wayback Machine, U.S. National Library of Medicine
- ^ "Sulfur Dioxide". Archived from the original on 2023-09-24. Retrieved 2024-03-22., U.S. National Library of Medicine
- ^ Lide DR, ed. (2006). CRC Handbook of Chemistry and Physics (87th ed.). Boca Raton, FL: CRC Press. ISBN 0-8493-0487-3.
- ^ "Hazardous Substances Data Bank".
- ^ Miller J Jr, Shah P, Yaws C (1976). "Correlation constants for chemical compounds". Chemical Engineering. 83 (25): 153–180. ISSN 0009-2460.
- ^ "C&L Inventory".
- ^ a b c NIOSH Pocket Guide to Chemical Hazards. "#0575". National Institute for Occupational Safety and Health (NIOSH).
- ^ a b "Sulfur dioxide". Immediately Dangerous to Life or Health Concentrations (IDLH). National Institute for Occupational Safety and Health (NIOSH).
- ^ a b Greenwood NN, Earnshaw A (1997). Chemistry of the Elements (2nd ed.). Butterworth-Heinemann. ISBN 978-0-08-037941-8.
- ^ a b Wothers P (2019). Antimony, Gold, and Jupiter's Wolf: How the Elements Were Named. Oxford University Press. ISBN 978-0-19-965272-3.
- ^ Cunningham, Terence P., Cooper, David L., Gerratt, Joseph, Karadakov, Peter B., Raimondi, Mario (1997). "Chemical bonding in oxofluorides of hypercoordinatesulfur". Journal of the Chemical Society, Faraday Transactions. 93 (13): 2247–2254. doi: 10.1039/A700708F.
- ^ US EPA O (2016-05-04). "Sulfur Dioxide Trends". www.epa.gov. Retrieved 2023-02-16.
- ^ Marcq E, Bertaux JL, Montmessin F, Belyaev D (2012). "Variations of sulphur dioxide at the cloud top of Venus's dynamic atmosphere". Nature Geoscience. 6 (1): 25–28. Bibcode: 2013NatGe...6...25M. doi: 10.1038/ngeo1650. ISSN 1752-0894. S2CID 59323909.
- ^ Halevy I, Zuber MT, Schrag DP (2007). "A Sulfur Dioxide Climate Feedback on Early Mars". Science. 318 (5858): 1903–1907. Bibcode: 2007Sci...318.1903H. doi: 10.1126/science.1147039. ISSN 0036-8075. PMID 18096802. S2CID 7246517.
- ^ Lellouch E (2007). "Io's atmosphere". In Lopes, R. M. C., Spencer, J. R. (eds.). Io after Galileo. Springer-Praxis. pp. 231–264. ISBN 978-3-540-34681-4.
- ^ "James Webb Space Telescope reveals an exoplanet atmosphere as never seen before".
- ^ Cruikshank DP, Howell RR, Geballe TR, Fanale FP (1985). "Sulfur Dioxide Ice on IO". ICES in the Solar System. pp. 805–815. doi: 10.1007/978-94-009-5418-2_55. ISBN 978-94-010-8891-6.
- ^ Europa's Hidden Ice Chemistry – NASA Jet Propulsion Laboratory. Jpl.nasa.gov (2010-10-04). Retrieved on 2013-09-24.
- ^ a b c Müller, Hermann. "Sulfur Dioxide". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi: 10.1002/14356007.a25_569. ISBN 978-3527306732.
- ^ Shriver, Atkins. Inorganic Chemistry, Fifth Edition. W. H. Freeman and Company; New York, 2010; p. 414.
- ^ WHITEHAVEN COAST ARCHAEOLOGICAL SURVEY. lakestay.co.uk (2007)
- ^ "Information archivée dans le Web" (PDF).
- ^ Zamboni CB, Medeiros IM, de Medeiros JA (October 2011). Analysis of Sulfur in Dried Fruits Using NAA (PDF). 2011 International Nuclear Atlantic Conference - INAC 2011. ISBN 978-85-99141-03-8. Archived from the original (PDF) on 2020-06-04. Retrieved 2020-06-04.
- ^ Current EU approved additives and their E Numbers, The Food Standards Agency website.
- ^ a b Preserving foods: Drying fruits and Vegetable (PDF), University of Georgia cooperative extension service, archived from the original (PDF) on 2022-09-27, retrieved 2022-06-06
- ^ "Practical Winery & vineyard Journal Jan/Feb 2009". www.practicalwinery.com. 1 Feb 2009. Archived from the original on 2013-09-28.
- ^ Sulphites in wine, MoreThanOrganic.com.
- ^ Jackson, R.S. (2008) Wine science: principles and applications, Amsterdam; Boston: Elsevier/Academic Press
- ^ Guerrero RF, Cantos-Villar E (2015). "Demonstrating the efficiency of sulphur dioxide replacements in wine: A parameter review". Trends in Food Science & Technology. 42: 27–43. doi: 10.1016/j.tifs.2014.11.004.
- ^ Chlorine Use in the Winery. Purdue University
- ^ Use of ozone for winery and environmental sanitation Archived 2017-09-12 at the Wayback Machine, Practical Winery & Vineyard Journal.
- ^ Tchobanoglous G (1979). Wastewater Engineering (3rd ed.). New York: McGraw Hill. ISBN 0-07-041677-X.
- ^ Engelmann L (July 2018). "Fumigating the Hygienic Model City: Bubonic Plague and the Sulfurozador in Early-Twentieth-Century Buenos Aires". Medical History. 62 (3): 360–382. doi: 10.1017/mdh.2018.37. PMC 6113751. PMID 29886876.
- ^ Liu D, Jin H, Tang C, Du J (2010). "Sulfur Dioxide: a Novel Gaseous Signal in the Regulation of Cardiovascular Functions". Mini-Reviews in Medicinal Chemistry. 10 (11): 1039–1045. doi: 10.2174/1389557511009011039. PMID 20540708.
- ^ Tian H (5 November 2014). "Advances in the study on endogenous sulfur dioxide in the cardiovascular system". Chinese Medical Journal. 127 (21): 3803–3807. doi: 10.3760/cma.j.issn.0366-6999.20133031. PMID 25382339. S2CID 11924999.
- ^ Yang R, Yang Y, Dong X, Wu X, Wei Y (Aug 2014). "Correlation between endogenous sulfur dioxide and homocysteine in children with pulmonary arterial hypertension associated with congenital heart disease". Zhonghua Er Ke Za Zhi (in Chinese). 52 (8): 625–629. PMID 25224243.
- ^ Liu D, Huang Y, Bu D, Liu AD, Holmberg L, Jia Y, Tang C, Du J, Jin H (May 2014). "Sulfur dioxide inhibits vascular smooth muscle cell proliferation via suppressing the Erk/MAP kinase pathway mediated by cAMP/PKA signaling". Cell Death Dis. 5 (5): e1251. doi: 10.1038/cddis.2014.229. PMC 4047873. PMID 24853429.
- ^ Wang XB, Jin HF, Tang CS, Du JB (16 Nov 2011). "The biological effect of endogenous sulfur dioxide in the cardiovascular system". Eur J Pharmacol. 670 (1): 1–6. doi: 10.1016/j.ejphar.2011.08.031. PMID 21925165.
- ^ Liang Y, Liu D, Ochs T, Tang C, Chen S, Zhang S, Geng B, Jin H, Du J (Jan 2011). "Endogenous sulfur dioxide protects against isoproterenol-induced myocardial injury and increases myocardial antioxidant capacity in rats". Lab. Invest. 91 (1): 12–23. doi: 10.1038/labinvest.2010.156. PMID 20733562.
- ^ Hoffman, R. V. (1990). "m-Trifluoromethylbenzenesulfonyl Chloride". Organic Syntheses; Collected Volumes, vol. 7, p. 508.
- ^ Olah GA, Lukas J (1967-08-01). "Stable carbonium ions. XLVII. Alkylcarbonium ion formation from alkanes via hydride (alkide) ion abstraction in fluorosulfonic acid-antimony pentafluoride-sulfuryl chlorofluoride solution". Journal of the American Chemical Society. 89 (18): 4739–4744. doi: 10.1021/ja00994a030. ISSN 0002-7863.
- ^ "Center for Science in the Public Interest – Chemical Cuisine". Retrieved March 17, 2010.
- ^ "California Department of Public Health: Food and Drug Branch: Sulfites" (PDF). Archived from the original (PDF) on July 23, 2012. Retrieved September 27, 2013.
- ^ Vally H, Misso NL (2012). "Adverse reactions to the sulphite additives". Gastroenterol Hepatol Bed Bench. 5 (1): 16–23. PMC 4017440. PMID 24834193.
- ^ Sulfur Dioxide Basics U.S. Environmental Protection Agency
- ^ a b Sulfur Dioxide (SO2) Pollution. United States Environmental Protection Agency
- ^ "NIOSH Pocket Guide to Chemical Hazards".
- ^ "Volcanic Sulfur Aerosols Affect Climate and the Earth's Ozone Layer". United States Geological Survey. Archived from the original on 14 November 2015. Retrieved 17 February 2009.
- ^ Mathera TA, Oppenheimer AG, McGonigle A (2004). "Aerosol chemistry of emissions from three contrasting volcanoes in Italy". Atmospheric Environment. 38 (33): 5637–5649. Bibcode: 2004AtmEn..38.5637M. doi: 10.1016/j.atmosenv.2004.06.017.
- ^ IPCC, 1990: Chapter 1: Greenhouse Gases and Aerosols [R.T. Watson, H. Rodhe, H. Oeschger and U. Siegenthaler]. In: Climate Change: The IPCC Scientific Assessment [J.T.Houghton, G.J.Jenkins and J.J.Ephraums (eds.)]. Cambridge University Press, Cambridge, United Kingdom and New York, NY, USA, pp. 31–34,
- ^ a b Effects of Acid Rain – Human Health Archived January 18, 2008, at the Wayback Machine. Epa.gov (June 2, 2006). Retrieved on 2013-02-09.
- ^ Henneman L, Choirat C, Dedoussi I, Dominici F, Roberts J, Zigler C (24 November 2023). "Mortality risk from United States coal electricity generation". Science. 382 (6673): 941.
- ^ Bates TS, Lamb BK, Guenther A, Dignon J, Stoiber RE (April 1992). "Sulfur emissions to the atmosphere from natural sources". Journal of Atmospheric Chemistry. 14 (1–4): 315–337. Bibcode: 1992JAtC...14..315B. doi: 10.1007/BF00115242. ISSN 0167-7764. S2CID 55497518.
- ^ Burns DA, Aherne J, Gay DA, Lehmann CM (2016). "Acid rain and its environmental effects: Recent scientific advances". Atmospheric Environment. 146: 1–4. Bibcode: 2016AtmEn.146....1B. doi: 10.1016/j.atmosenv.2016.10.019.
- ^ "Effects of Acid Rain - Surface Waters and Aquatic Animals". US EPA. Archived from the original on 14 May 2009.
- ^ Rodhe H, Dentener F, Schulz M (2002-10-01). "The Global Distribution of Acidifying Wet Deposition". Environmental Science & Technology. 36 (20): 4382–4388. Bibcode: 2002EnST...36.4382R. doi: 10.1021/es020057g. ISSN 0013-936X. PMID 12387412.
- ^ US EPA: Effects of Acid Rain – Forests Archived July 26, 2008, at the Wayback Machine
- ^ Likens GE, Driscoll CT, Buso DC (1996). "Long-Term Effects of Acid Rain: Response and Recovery of a Forest Ecosystem" (PDF). Science. 272 (5259): 244. Bibcode: 1996Sci...272..244L. doi: 10.1126/science.272.5259.244. S2CID 178546205. Archived (PDF) from the original on December 24, 2012. Retrieved February 9, 2013.
- ^ Larssen T, Carmichael GR (2000-10-01). "Acid rain and acidification in China: the importance of base cation deposition". Environmental Pollution. 110 (1): 89–102. doi: 10.1016/S0269-7491(99)00279-1. ISSN 0269-7491. PMID 15092859. Archived from the original on March 30, 2015. Retrieved April 22, 2020.
- ^ Johnson DW, Turner J, Kelly JM (1982). "The effects of acid rain on forest nutrient status". Water Resources Research. 18 (3): 449–461. Bibcode: 1982WRR....18..449J. doi: 10.1029/WR018i003p00449. ISSN 1944-7973.
- ^ Wang X, Ding H, Ryan L, Xu X (1 May 1997). "Association between air pollution and low birth weight: a community-based study". Environmental Health Perspectives. 105 (5): 514–20. doi: 10.1289/ehp.97105514. ISSN 0091-6765. PMC 1469882. PMID 9222137. S2CID 2707126.
- ^ Xu Y, Ramanathan V, Victor DG (5 December 2018). "Global warming will happen faster than we think". Nature. 564 (7734): 30–32. Bibcode: 2018Natur.564...30X. doi: 10.1038/d41586-018-07586-5. PMID 30518902.
- ^ Lin CK, Lin RT, Chen PC, Wang P, De Marcellis-Warin N, Zigler C, Christiani DC (2018-02-08). "A Global Perspective on Sulfur Oxide Controls in Coal-Fired Power Plants and Cardiovascular Disease". Scientific Reports. 8 (1): 2611. Bibcode: 2018NatSR...8.2611L. doi: 10.1038/s41598-018-20404-2. ISSN 2045-2322. PMC 5805744. PMID 29422539.
- ^ Lindeburg MR (2006). Mechanical Engineering Reference Manual for the PE Exam. Belmont, C.A.: Professional Publications, Inc. pp. 27–3. ISBN 978-1-59126-049-3.
- ^ FAQ's About Sulfur Removal and Recovery using the LO-CAT® Hydrogen Sulfide Removal System. gtp-merichem.com
- ^ Process screening analysis of alternative gas treating and sulfur removal for gasification. (December 2002) Report by SFA Pacific, Inc. prepared for U.S. Department of Energy (PDF) Retrieved on 2011-10-31.
- ^ May, Walter R. Marine Emissions Abatement Archived 2015-04-02 at the Wayback Machine. SFA International, Inc., p. 6.
- ^ a b Julsrud IR, Storelvmo T, Schulz M, Moseid KO, Wild M (20 October 2022). "Disentangling Aerosol and Cloud Effects on Dimming and Brightening in Observations and CMIP6". Journal of Geophysical Research: Atmospheres. 127 (21): e2021JD035476. Bibcode: 2022JGRD..12735476J. doi: 10.1029/2021JD035476.
- ^ Stanhill G, Moreshet S (6 November 2004). "Global radiation climate changes in Israel". Climatic Change. 22 (2): 121–138. Bibcode: 1992ClCh...22..121S. doi: 10.1007/BF00142962. S2CID 154006620.
- ^ Gilgen H, Wild M, Ohmura A (1998). "Means and trends of shortwave irradiance at the surface estimated from global energy balance archive data" (PDF). Journal of Climate. 11 (8): 2042–2061. Bibcode: 1998JCli...11.2042G. doi: 10.1175/1520-0442-11.8.2042.
- ^ Stanhill G, Cohen S (2001). "Global dimming: a review of the evidence for a widespread and significant reduction in global radiation with discussion of its probable causes and possible agricultural consequences". Agricultural and Forest Meteorology. 107 (4): 255–278. Bibcode: 2001AgFM..107..255S. doi: 10.1016/S0168-1923(00)00241-0.
- ^ Liepert BG (2 May 2002). "Observed Reductions in Surface Solar Radiation in the United States and Worldwide from 1961 to 1990" (PDF). Geophysical Research Letters. 29 (12): 61–1–61–4. Bibcode: 2002GeoRL..29.1421L. doi: 10.1029/2002GL014910.
-
^ Eddy JA, Gilliland RL, Hoyt DV (23 December 1982). "Changes in the solar constant and climatic effects".
Nature. 300 (5894): 689–693.
Bibcode:
1982Natur.300..689E.
doi:
10.1038/300689a0.
S2CID
4320853.
Spacecraft measurements have established that the total radiative output of the Sun varies at the 0.1−0.3% level
- ^ a b "Aerosol pollution has caused decades of global dimming". American Geophysical Union. 18 February 2021. Archived from the original on 27 March 2023. Retrieved 18 December 2023.
- ^ Adam D (18 December 2003). "Goodbye sunshine". The Guardian. Retrieved 26 August 2009.
- ^ Wild M, Wacker S, Yang S, Sanchez-Lorenzo A (1 February 2021). "Evidence for Clear-Sky Dimming and Brightening in Central Europe". Geophysical Research Letters. 48 (6). Bibcode: 2021GeoRL..4892216W. doi: 10.1029/2020GL092216. hdl: 20.500.11850/477374. S2CID 233645438.
- ^ a b Xu Y, Ramanathan V, Victor DG (5 December 2018). "Global warming will happen faster than we think". Nature. 564 (7734): 30–32. Bibcode: 2018Natur.564...30X. doi: 10.1038/d41586-018-07586-5. PMID 30518902.
- ^ Bellouin N, Quaas J, Gryspeerdt E, Kinne S, Stier P, Watson-Parris D, Boucher O, Carslaw KS, Christensen M, Daniau AL, Dufresne JL, Feingold G, Fiedler S, Forster P, Gettelman A, Haywood JM, Lohmann U, Malavelle F, Mauritsen T, McCoy DT, Myhre G, Mülmenstädt J, Neubauer D, Possner A, Rugenstein M, Sato Y, Schulz M, Schwartz SE, Sourdeval O, Storelvmo T, Toll V, Winker D, Stevens B (1 November 2019). "Bounding Global Aerosol Radiative Forcing of Climate Change". Reviews of Geophysics. 58 (1): e2019RG000660. doi: 10.1029/2019RG000660. PMC 7384191. PMID 32734279.
- ^ IPCC, 2021: Summary for Policymakers. In: Climate Change 2021: The Physical Science Basis. Contribution of Working Group I to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change [Masson-Delmotte, V., P. Zhai, A. Pirani, S.L. Connors, C. Péan, S. Berger, N. Caud, Y. Chen, L. Goldfarb, M.I. Gomis, M. Huang, K. Leitzell, E. Lonnoy, J.B.R. Matthews, T.K. Maycock, T. Waterfield, O. Yelekçi, R. Yu, and B. Zhou (eds.)]. Cambridge University Press, Cambridge, United Kingdom and New York, NY, USA, pp. 3–32, doi: 10.1017/9781009157896.001.
- ^ Hausfather Z (29 April 2021). "Explainer: Will global warming 'stop' as soon as net-zero emissions are reached?". Carbon Brief. Retrieved 3 March 2023.
- ^ Hassan T, Allen RJ, et al. (27 June 2022). "Air quality improvements are projected to weaken the Atlantic meridional overturning circulation through radiative forcing effects". Communications Earth & Environment. 3 (3): 149. Bibcode: 2022ComEE...3..149H. doi: 10.1038/s43247-022-00476-9. S2CID 250077615.
- ^ "Trends in Atmospheric Methane". NOAA. Retrieved 14 October 2022.
- ^ Tollefson J (8 February 2022). "Scientists raise alarm over 'dangerously fast' growth in atmospheric methane". Nature. Retrieved 14 October 2022.
- ^ Lan X, Basu S, Schwietzke S, Bruhwiler LM, Dlugokencky EJ, Michel SE, Sherwood OA, Tans PP, Thoning K, Etiope G, Zhuang Q, Liu L, Oh Y, Miller JB, Pétron G, Vaughn BH, Crippa M (8 May 2021). "Improved Constraints on Global Methane Emissions and Sinks Using δ13C-CH4". Global Biogeochemical Cycles. 35 (6): e2021GB007000. Bibcode: 2021GBioC..3507000L. doi: 10.1029/2021GB007000. PMC 8244052. PMID 34219915.
- ^ Feng L, Palmer PI, Zhu S, Parker RJ, Liu Y (16 March 2022). "Tropical methane emissions explain large fraction of recent changes in global atmospheric methane growth rate". Nature Communications. 13 (1): 1378. Bibcode: 2022NatCo..13.1378F. doi: 10.1038/s41467-022-28989-z. PMC 8927109. PMID 35297408.
- ^ Quaas J, Jia H, Smith C, Albright AL, Aas W, Bellouin N, Boucher O, Doutriaux-Boucher M, Forster PM, Grosvenor D, Jenkins S, Klimont Z, Loeb NG, Ma X, Naik V, Paulot F, Stier P, Wild M, Myhre G, Schulz M (21 September 2022). "Robust evidence for reversal of the trend in aerosol effective climate forcing". Atmospheric Chemistry and Physics. 22 (18): 12221–12239. Bibcode: 2022ACP....2212221Q. doi: 10.5194/acp-22-12221-2022. hdl: 20.500.11850/572791. S2CID 252446168.
- ^ Kobayashi Y, Ide Y, Takegawa N (2021-04-03). "Development of a novel particle mass spectrometer for online measurements of refractory sulfate aerosols". Aerosol Science and Technology. 55 (4): 371–386. Bibcode: 2021AerST..55..371K. doi: 10.1080/02786826.2020.1852168. ISSN 0278-6826. S2CID 229506768.
- ^ Palumbo, P., A. Rotundi, V. Della Corte, A. Ciucci, L. Colangeli, F. Esposito, E. Mazzotta Epifani, V. Mennella, J.R. Brucato, F.J.M. Rietmeijer, G. J. Flynn, J.-B. Renard, J.R. Stephens, E. Zona. "The DUSTER experiment: collection and analysis of aerosol in the high stratosphere". Retrieved 19 February 2009.[ permanent dead link]
- ^ Myhre G, Stordal F, Berglen TF, Sundet JK, Isaksen IS (2004-03-01). "Uncertainties in the Radiative Forcing Due to Sulfate Aerosols". Journal of the Atmospheric Sciences. 61 (5): 485–498. Bibcode: 2004JAtS...61..485M. doi: 10.1175/1520-0469(2004)061<0485:UITRFD>2.0.CO;2. ISSN 0022-4928. S2CID 55623817.
- ^ Zhang J, Furtado K, Turnock ST, Mulcahy JP, Wilcox LJ, Booth BB, Sexton D, Wu T, Zhang F, Liu Q (22 December 2021). "The role of anthropogenic aerosols in the anomalous cooling from 1960 to 1990 in the CMIP6 Earth system models". Atmospheric Chemistry and Physics. 21 (4): 18609–18627. Bibcode: 2021ACP....2118609Z. doi: 10.5194/acp-21-18609-2021.
- ^ "Aerosols and Incoming Sunlight (Direct Effects)". NASA. 2 November 2010.
- ^ "Stratospheric Injections Could Help Cool Earth, Computer Model Shows". ScienceDaily. 15 September 2006. Retrieved 19 February 2009.
- ^ Launder B., J.M.T. Thompson (1996). "Global and Arctic climate engineering: numerical model studies". Phil. Trans. R. Soc. A. 366 (1882): 4039–56. Bibcode: 2008RSPTA.366.4039C. doi: 10.1098/rsta.2008.0132. PMID 18757275.
- ^ Crutzen PJ (2006). "Albedo Enhancement by Stratospheric Sulfur Injections: A Contribution to Resolve a Policy Dilemma?". Climatic Change. 77 (3–4): 211–220. Bibcode: 2006ClCh...77..211C. doi: 10.1007/s10584-006-9101-y.
- ^ Visioni D, Slessarev E, MacMartin DG, Mahowald NM, Goodale CL, Xia L (1 September 2020). "What goes up must come down: impacts of deposition in a sulfate geoengineering scenario". Environmental Research Letters. 15 (9): 094063. Bibcode: 2020ERL....15i4063V. doi: 10.1088/1748-9326/ab94eb. ISSN 1748-9326.
- ^ Andrew Charlton-Perez, Eleanor Highwood. "Costs and benefits of geo-engineering in the Stratosphere" (PDF). Archived from the original (PDF) on 14 January 2017. Retrieved 17 February 2009.
- ^ Trisos CH, Geden O, Seneviratne SI, Sugiyama M, van Aalst M, Bala G, Mach KJ, Ginzburg V, de Coninck H, Patt A (2021). "Cross-Working Group Box SRM: Solar Radiation Modification" (PDF). Climate Change 2021: The Physical Science Basis. Contribution of Working Group I to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change. 2021: 1238. Bibcode: 2021AGUFM.U13B..05K. doi: 10.1017/9781009157896.007.
- ^ Holman JP (2002). Heat Transfer (9th ed.). New York, NY: McGraw-Hill Companies, Inc. pp. 600–606. ISBN 9780072406559.
- ^ Incropera rP, Dewitt DP, Bergman TL, Lavigne AS (2007). Fundamentals of Heat and Mass Transfer (6th ed.). Hoboken, NJ: John Wiley and Sons, Inc. pp. 941–950. ISBN 9780471457282.
External links
- Global map of sulfur dioxide distribution
- United States Environmental Protection Agency Sulfur Dioxide page
- International Chemical Safety Card 0074
- IARC Monographs. "Sulfur Dioxide and some Sulfites, Bisulfites and Metabisulfites". vol. 54. 1992. p. 131.
- NIOSH Pocket Guide to Chemical Hazards
- CDC – Sulfure Dioxide – NIOSH Workplace Safety and Health Topic
- Sulfur Dioxide, Molecule of the Month