 | |
| Clinical data | |
|---|---|
| Trade names | Canespor, many others |
| AHFS/ Drugs.com | International Drug Names |
|
Routes of administration | Topical |
| ATC code | |
| Legal status | |
| Legal status |
|
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard ( EPA) | |
| ECHA InfoCard | 100.056.651 |
| Chemical and physical data | |
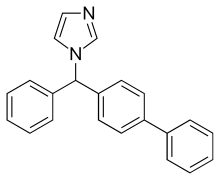
| Formula | C22H18N2 |
| Molar mass | 310.400 g·mol−1 |
| 3D model ( JSmol) | |
| Chirality | Racemic mixture |
| |
| |
| | |
Bifonazole (trade name Canespor among others [1]) is an imidazole antifungal drug used in form of ointments.
It was patented in 1974 and approved for medical use in 1983. [2] There are also combinations with carbamide for the treatment of onychomycosis.
Adverse effects
The most common side effect is a burning sensation at the application site. Other reactions, such as itching, eczema or skin dryness, are rare. [3] Bifonazole is a potent aromatase inhibitor in vitro. [4] [5]
Pharmacology
Mechanism of action
Bifonazole has a dual mode of action. It inhibits fungal ergosterol biosynthesis at two points, via transformation of 24-methylendihydrolanosterol to desmethylsterol, together with inhibition of HMG-CoA. This enables fungicidal properties against dermatophytes and distinguishes bifonazole from other antifungal drugs. [3] [6]
Pharmacokinetics
Six hours after application, bifonazole concentrations range from 1000 μg/ cm3 in the stratum corneum to 5 μg/cm3 in the papillary dermis. [3]
References
- ^ International Drug Names: Bifonazole.
- ^ Fischer J, Ganellin CR (2006). Analogue-based Drug Discovery. John Wiley & Sons. p. 502. ISBN 9783527607495.
- ^ a b c Haberfeld H, ed. (2015). Austria-Codex (in German). Vienna: Österreichischer Apothekerverlag. Canesten Bifonazol-Creme.
- ^ Trösken ER, Fischer K, Völkel W, Lutz WK (February 2006). "Inhibition of human CYP19 by azoles used as antifungal agents and aromatase inhibitors, using a new LC-MS/MS method for the analysis of estradiol product formation". Toxicology. 219 (1–3): 33–40. doi: 10.1016/j.tox.2005.10.020. PMID 16330141.
- ^ Egbuta C, Lo J, Ghosh D (December 2014). "Mechanism of inhibition of estrogen biosynthesis by azole fungicides". Endocrinology. 155 (12): 4622–4628. doi: 10.1210/en.2014-1561. PMC 4239419. PMID 25243857.
- ^ Berg D, Regel E, Harenberg HE, Plempel M (1984). "Bifonazole and clotrimazole. Their mode of action and the possible reason for the fungicidal behaviour of bifonazole". Arzneimittel-Forschung. 34 (2): 139–146. PMID 6372801.