|
| This It is of interest to the following WikiProjects: | ||||||||||
| |||||||||||
| This is the
talk page for discussing improvements to the
Atomic nucleus article. This is not a forum for general discussion of the article's subject. |
Article policies
|
| Find sources: Google ( books · news · scholar · free images · WP refs) · FENS · JSTOR · TWL |
| Archives: 1 |
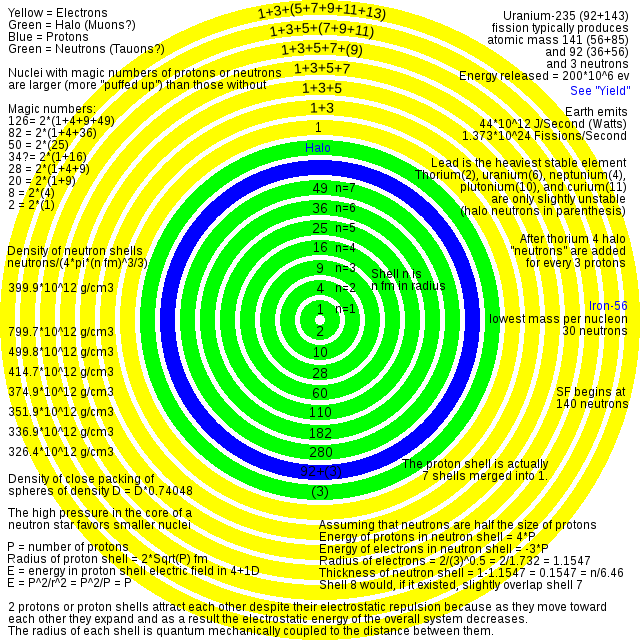
article states:
The stable nucleus has approximately a constant density and therefore the nuclear radius R can be approximated by the following formula, where A = Atomic mass number (the number of protons Z, plus the number of neutrons N) and r0 = 1.25 fm = 1.25 × 10−15 m. In this equation, the constant r0 varies by 0.2 fm, depending on the nucleus in question, but this is less than 20% change from a constant.
I believe I can explain why the fudge factor varies by 20% (from 1 to 1.35)
https://upload.wikimedia.org/wikipedia/commons/d/d1/Uranium_atom.svg
Just granpa ( talk) 02:21, 18 January 2017 (UTC)
- That sounds like WP:original research, is this publiched clearly somehwhere on a reliable source? Graeme Bartlett ( talk) 01:56, 20 July 2018 (UTC)
Categories:
- B-Class vital articles
- Wikipedia level-4 vital articles
- Wikipedia vital articles in Physical sciences
- B-Class level-4 vital articles
- Wikipedia level-4 vital articles in Physical sciences
- B-Class vital articles in Physical sciences
- B-Class physics articles
- Top-importance physics articles
- B-Class physics articles of Top-importance