| |
| Identifiers | |
|---|---|
3D model (
JSmol)
|
|
| |
| |
| Properties | |
| C2H7NO4S | |
| Molar mass | 141.14 g·mol−1 |
| Related compounds | |
Related compounds
|
Taurine |
Except where otherwise noted, data are given for materials in their
standard state (at 25 °C [77 °F], 100 kPa).
| |
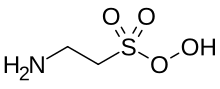
Peroxytaurine appears to be the underresearched product of superoxide oxidation of taurine, including in cooked food. [1]
References
- ^ Grove, Roxanna Q.; Karpowicz, Steven J. (2017). "Reaction of hypotaurine or taurine with superoxide produces the organic peroxysulfonic acid peroxytaurine". Free Radical Biology and Medicine. 108: 575–584. doi: 10.1016/j.freeradbiomed.2017.04.342. PMID 28438660. S2CID 4722763.