| |
| Names | |
|---|---|
|
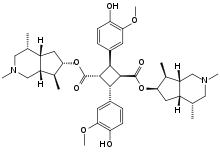
Preferred IUPAC name
Bis[(4R,4aS,6R,7S,7aR)-2,4,7-trimethyloctahydro-1H-cyclopenta[c]pyridin-6-yl] (1R,2R,3S,4S)-2,4-bis(4-hydroxy-3-methoxyphenyl)cyclobutane-1,3-dicarboxylate | |
| Identifiers | |
3D model (
JSmol)
|
|
| ChEMBL | |
| ChemSpider | |
PubChem
CID
|
|
| |
| |
| Properties | |
| C42H58N2O8 | |
| Molar mass | 718.932 g·mol−1 |
Except where otherwise noted, data are given for materials in their
standard state (at 25 °C [77 °F], 100 kPa).
| |
Incarvillateine is a complex monoterpene alkaloid that is a derivative of α- truxillic acid. It can be isolated from the plant genus Incarvillea.
Biological activity
Opioidergic
Incarvillateine isolated from Incarvillea sinensis has demonstrated significant analgesic activity when compared to the opiate alkaloid morphine. [1]
Incarvillateine's pain-killing effect was partially blocked by administration of naloxone, [2] norbinaltorphimine and beta-funaltrexamine, [3] which are receptor antagonists with varying selectivity for mu and kappa opioid receptors. Naltrindole, a delta opioid receptor antagonist, did not counteract the analgesic activity of incarvillateine. [3]
These findings indicate that incarvillateine may possess opioidergic receptor activity, but it is worthy to note that some studies indicate that naloxone was ineffective at countering incarvillateine's analgesic activity. [4]
Adenosinergic
Incarvillateine's antinociceptive effect was blocked by the administration of adenosine receptor antagonists such as theophylline. This suggests that incarvillateine's main mechanism of action is mediated through the adenosine receptor. [4]
References
- ^ Nakamura, M.; Chi, Y. M.; Yan, W. M.; Nakasugi, Y.; Yoshizawa, T.; Irino, N.; Hashimoto, F.; Kinjo, J.; Nohara, T. (1999-09-01). "Strong antinociceptive effect of incarvillateine, a novel monoterpene alkaloid from Incarvillea sinensis". Journal of Natural Products. 62 (9): 1293–1294. doi: 10.1021/np990041c. ISSN 0163-3864. PMID 10514316.
- ^ Ichikawa, Masaya; Takahashi, Masaki; Aoyagi, Sakae; Kibayashi, Chihiro (2004). "Total Synthesis of (−)-Incarvilline, (+)-Incarvine C, and (−)-Incarvillateine". Journal of the American Chemical Society. 126 (50): 16553–16558. doi: 10.1021/ja0401702. PMID 15600360.
- ^ a b Chi, Yu-Ming; Nakamura, Motoyuki; Yoshizawa, Toyokichi; Zhao, Xi-Ying; Yan, Wen-Mei; Hashimoto, Fumio; Kinjo, Junei; Nohara, Toshihiro; Sakurada, Shinobu (2005-10-01). "Pharmacological study on the novel antinociceptive agent, a novel monoterpene alkaloid from Incarvillea sinensis". Biological & Pharmaceutical Bulletin. 28 (10): 1989–1991. doi: 10.1248/bpb.28.1989. ISSN 0918-6158. PMID 16204962.
- ^ a b Wang, Mei-Liang; Yu, Gang; Yi, Shou-Pu; Zhang, Feng-Ying; Wang, Zhi-Tong; Huang, Bin; Su, Rui-Bin; Jia, Yan-Xing; Gong, Ze-Hui (2015-11-03). "Antinociceptive effects of incarvillateine, a monoterpene alkaloid from Incarvillea sinensis, and possible involvement of the adenosine system". Scientific Reports. 5: 16107. Bibcode: 2015NatSR...516107W. doi: 10.1038/srep16107. ISSN 2045-2322. PMC 4630779. PMID 26527075.