(Redirected from
1,2-Dimethylethylenediamine)
| |
| Names | |
|---|---|
|
Preferred IUPAC name
N,N′-Dimethylethane-1,2-diamine | |
| Other names
N,N′-Dimethyl-1,2-ethanediamine
| |
| Identifiers | |
3D model (
JSmol)
|
|
| ChemSpider | |
| ECHA InfoCard | 100.003.450 |
| EC Number |
|
PubChem
CID
|
|
| UNII | |
CompTox Dashboard (
EPA)
|
|
| |
| |
| Properties | |
| C4H12N2 | |
| Molar mass | 88.154 g·mol−1 |
| Appearance | Colorless liquid |
| Density | 0.819 g/mL |
| Boiling point | 120 °C (248 °F; 393 K) |
Except where otherwise noted, data are given for materials in their
standard state (at 25 °C [77 °F], 100 kPa).
| |
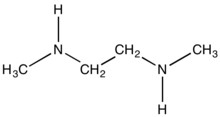
N,N'-Dimethylethylenediamine (DMEDA) is the organic compound with the formula (CH3NH)2C2H4. It is a colorless liquid with a fishy odor. It features two secondary amine functional groups. Regarding its name, N and N' indicate that the methyl groups are attached to different nitrogen atoms.
Reactions
DMEDA is used as a chelating diamine for the preparation of metal complexes, some of which function as homogeneous catalysts. [1] [2]
The compound is used as a precursor to imidazolidines by condensation with ketones or with aldehydes:
- O=CRR' + C2H4[NH(CH3)]2 → C2H4[NH(CH3)]2CRR' + H2O

DMEDA complexes of copper(I) halides are used to catalyze C-N coupling reactions. [4]
See also
References
- ^ Chan, Timothy R.; Hilgraf, Robert; Sharpless, K. Barry; Fokin, Valery V. "Polytriazoles as copper(I)-stabilizing ligands in catalysis" Organic Letters 2004, volume 6, 2853-2855. doi: 10.1021/ol0493094
- ^ Klapars, Artis; Huang, Xiaohua; Buchwald, Stephen L. (2002). "A General and Efficient Copper Catalyst for the Amidation of Aryl Halides". Journal of the American Chemical Society. 124 (25): 7421–7428. doi: 10.1021/ja0260465. PMID 12071751.
- ^ Ariyananda, W. G. Piyal; Norman, Richard E. (2006). "trans-Dichlorobis(N,N'-dimethylethane-1,2-diamine-κ2N,N')nickel(II)". Acta Crystallographica Section E. 62: m2339–m2341. doi: 10.1107/S1600536806033770.
- ^ Krout, M. R.; Mohr, J. T.; Stoltz, B. M. (2009). "PREPARATION OF (S)-tert-ButylPHOX". Organic Syntheses. 86: 181. doi: 10.15227/orgsyn.086.0181. PMC 2805096. PMID 20072718.